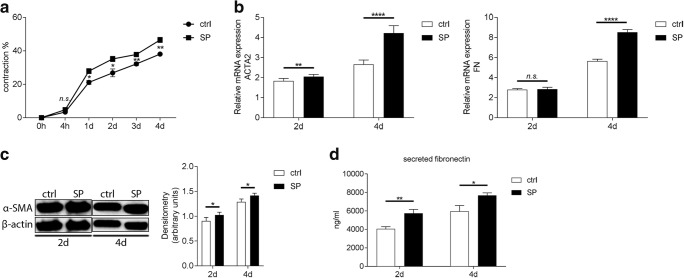
Fig. 3.
SP increases contractile abilities, and upregulates gene expression, protein secretion, and expression of fibrotic markers fibronectin and α-SMA in corneal fibroblasts during the onset on fibrosis in vitro. a Bovine collagen I liquid gel was mixed together with 0.25 × 106 corneal fibroblasts, FBS (10%), 0.5 mM VitC, and 0.25 ng/ml recombinant human TGF-β1. Experimental groups were treated with 10−5M SP. Gels containing fibroblasts, 10% FBS, VitC, and TGF-β1 served as positive controls for the fibrosis model (ctrl) and treated gels were compared with them. Gel contraction assay showed that 10−5M SP increased contractile abilities of corneal fibroblasts already at day 1 when compared with positive control. b RT-qPCR showed that 10−5M SP upregulated ACTA2 gene expression in corneal fibroblasts both 2 days and 4 days after treatment and induction of fibrosis. Gene expression of FN was unaffected by SP treatment at day 2; however, it was significantly increased 4 days after treatment. c α-SMA protein expression was assessed by western blot at 2 days and 4 days after treatment with 10−5M SP and induction of fibrosis. Densitometry analysis was performed in order to quantify the western blot results. Intensity of α-SMA band was normalized to the intensity of β-actin band. SP treatment resulted in increased α-SMA (42 kDa) protein expression both 2 days and 4 days after treatment. β-actin (45 kDa) served as loading control. d Supernatants collected 2 days and 4 days after induction of fibrosis and treatment with 10−5M SP were subjected to fibronectin ELISA. Secretion of fibronectin was increased in supernatants collected from SP-treated cells both 2 days and 4 days after treatment. Values are means ± SD. n.s. (not significant); *p < 0.05; **p < 0.01; ****p < 0.0001