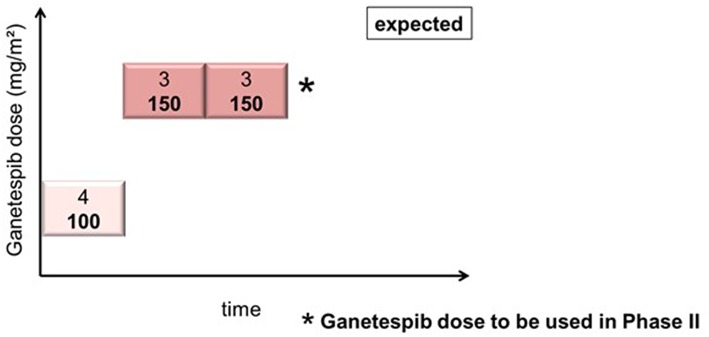
Figure 1.
Actual course of the GANNET53 phase I dose escalation/de-escalation trial. Boxes depict patient cohorts and provide information on the number on patients (4 in cohort 1, and 3 in cohorts 2 and 3, respectively) and the dose level of ganetespib (100 mg/m2 in cohort 1, and 150 mg/m2 in cohorts 2 and 3, respectively). The actual course of the trial took place as expected with one dose escalation step and without necessity for dose de-escalation due to lack of dose-limiting toxicities (DLTs) in the DLT observation time frame of cycles 1 and 2.