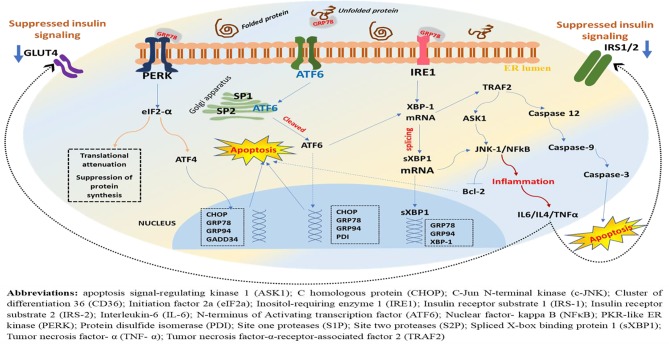
Figure 2.
A schematic representation of downstream effectors, target genes, and possible outcome in the ERS-activated UPR. Under ERS, dissociation of GRP78 from its luminal domain leads to oligomerization and autophosphorylation of PERK, ensuing its kinase and endoribonuclease activities. As a result, the α subunit of eukaryotic initiation factor 2 (eIF2) undergoes phosphorylation, resulting in translational attenuation characterized by a reduction in protein biosynthesis. Parallelly, this downstream phosphorylation of eIF2 leads to increased expression of ATF4 and translocation into the nucleus where it binds to the UPR element (UPRE) resulting in transcriptional modification of UPR target genes including the proapoptotic transcription factor, C homologous protein (CHOP), GRP78, GRP94, and GADD34, while attenuating global translational process, but PERK phosphorylation also inhibits transcription of I kappa B alpha (IκBα), leading to hyperactivation of NF-κB and increased production of inflammatory cytokines ( Figure 4 ). On the other hand, ERS leads to autophosphorylation of IRE-1, leading to excision and splicing of its substrate, XBP1 mRNA. Consequently, this results in spliced XBP1 protein (sXBP1), which translocates into the nucleus, and this will upregulate genes for protein folding enzymes secretion and ER-associated protein degradation (ERAD). Noteworthy, the ATF6 is activated following PERK and prior to IRE1. The GRP78 dissociates from ATF6 and recruited to luminal protein aggregates resulting in translocation of ATF6’s cytosolic fraction to the Golgi, where it is spliced and proteolyzed by site one proteases (S1Ps) and site two proteases (S2Ps). Eventually, this leads to the release of the cytosolic domain of ATF6 and entry into the nucleus where it sequesters with the ERS response element (ERSE) resulting in the activation of UPR target genes including XBP1, CHOP, and GRP78/BiP, GRP94, PDI. However, chronic ERS, which may result from persistent perturbation of the ER, can lead to malfunction within the network and failure of the UPR adaptive system. And as a result, the PERK-mediated post-translational attenuation is inhibited and further accumulations of unfolded protein aggregates, leading to initiation of C homologous protein (CHOP)-mediated apoptotic deaths of the cells.