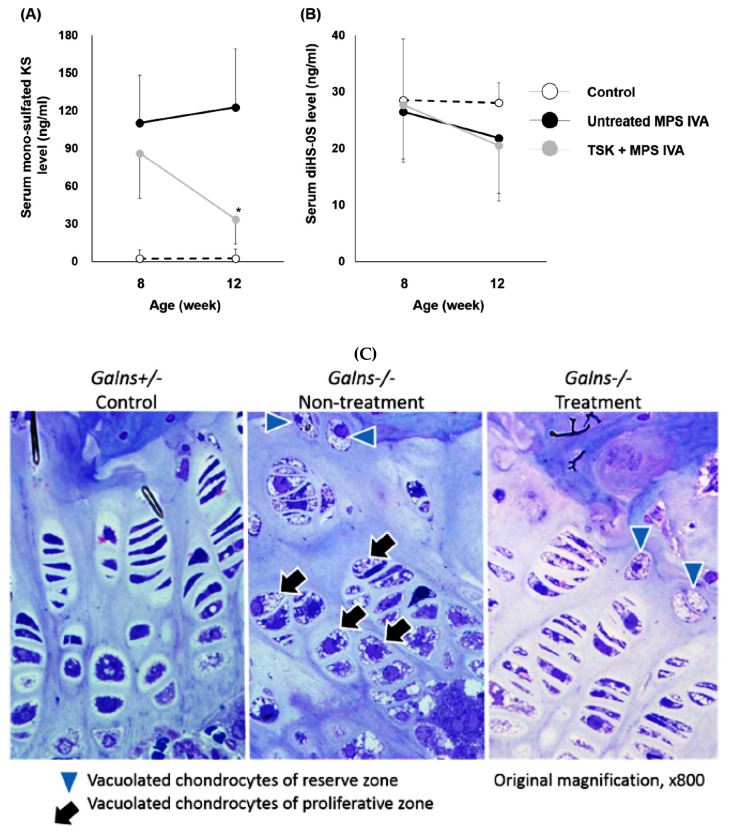
Figure 3.
Serum glycosaminoglycan levels and bone pathology of MPS IVA mice after repeated administration of thermostable keratanase. MPS IVA mice were treated with 2 U/kg of thermostable keratanase intravenously 3 times at 0, 4, and 8 weeks of age, and PBS was administered into MPS IVA mice (untreated) and heterozygous mice (control) in the same manner. Serum samples were collected from the superficial temporal vein at the time points of 8 and 12 weeks of age. Tissues were collected at 12 weeks of age. (A,B) The level of serum mono-sulfated KS and diHS-0S was measured by LC-MS/MS. n = 7–9. (C) Bone pathology in growth plate regions of femur and tibia was evaluated by toluidine blue staining. Statistics were analyzed by one-way ANOVA with the Bonferroni’s post-hoc test. Data are presented as mean ± SD. *p < 0.05 vs. untreated MPS IVA. HS: heparan sulfate, KS: keratan sulfate, TSK: thermostable keratanase.