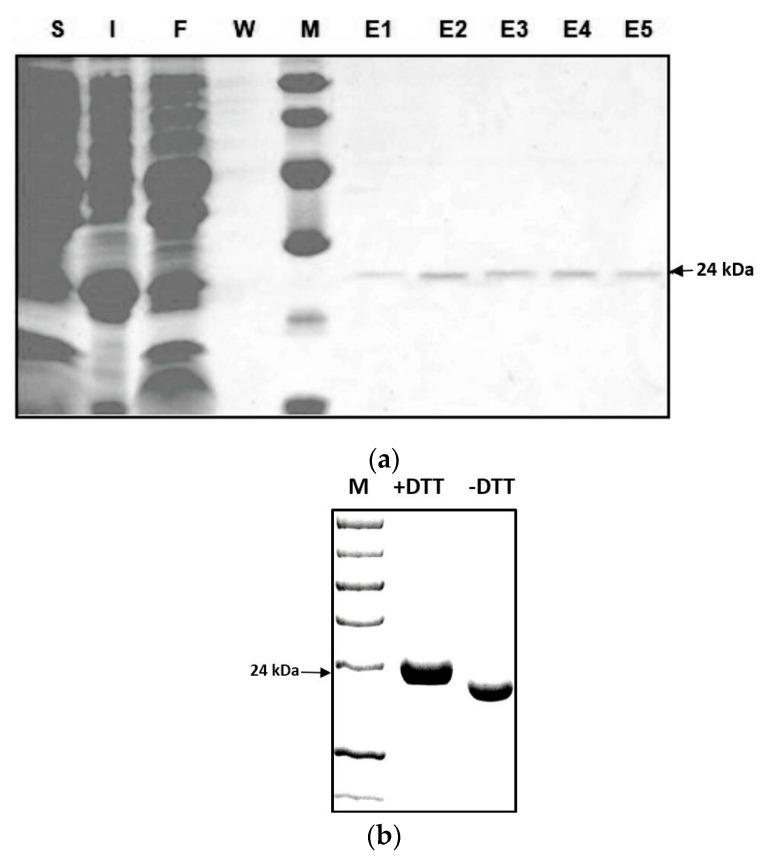
Figure 3.
(a) SDS–PAGE of the recombinant Zm-INVINH4 protein in E.coli with a predicted mass of 24 kDa. Soluble proteins (S); insoluble proteins (I); flow-through of the column (F); wash fraction (W); and elution (E1–5). (b) The increased mobility of the recombinant Zm-INVINH4 protein during gel electrophoresis in the absence of a reductant, as compared with the reductant (DTT)-containing sample buffer, which indicates that the active conformation forms a rather compact structure.