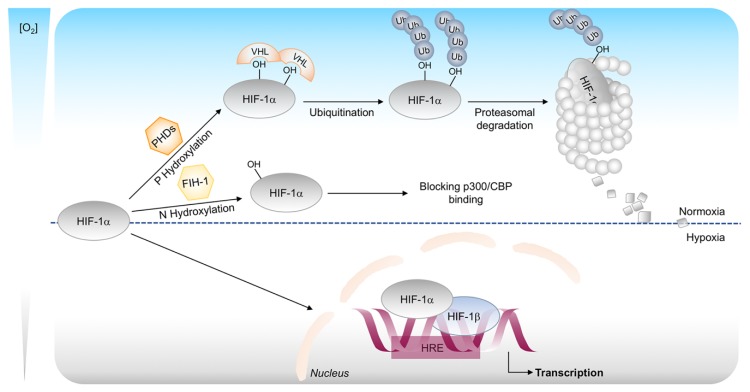
Figure 2.
Canonical regulation of HIF-1α stability. In oxygenated conditions, HIF-1α is hydroxylated on proline residues by prolyl-4-hydroxylases (PHDs) and polyubiquitinated by the von Hippel–Lindau protein (pVHL). This leads to degradation of HIF-1α by the 26S proteasome system. The oxygen-dependent hydroxylation of asparagine residue by the enzyme FIH-1 impairs p300 and CBP binding and inhibits HIF-1 mediated gene transcription. In hypoxic conditions, HIF-1α is stabilized and translocated into the nucleus, where it binds to its dimerization partner HIF-1β and enhances the transcription of HIF target genes.