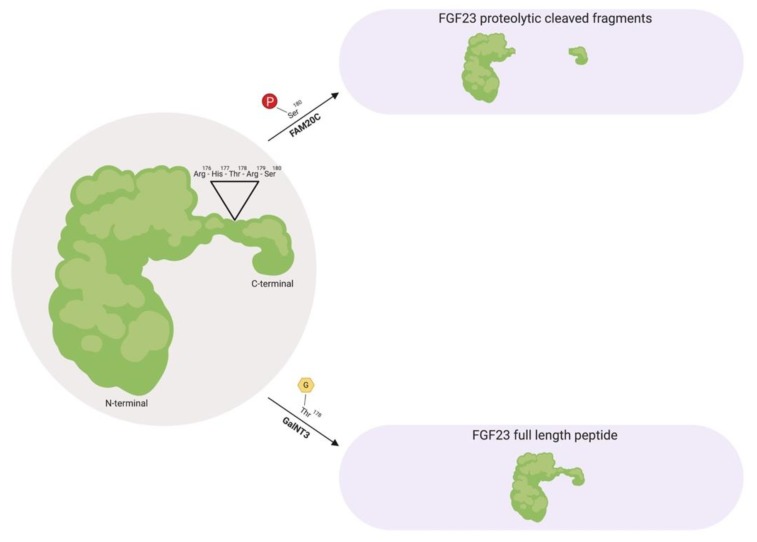
Figure 1.
Fibroblast growth factor 23 (FGF23) regulation by post-translational modification. As a full-length biologically active peptide in circulation, FGF23 is O-glycosylated by GalNT3 at several residues such as Thr178, which protects FGF23 from proteolytic cleavage by pro-protein convertases such as furin. Vice versa, as proteolytic cleaved fragments in circulation, FAM20C phosphorylates FGF23 at multiple amino acids, such as Ser180. This phosphorylation event impedes O-glycosylation by GalNT3 and allows furin to recognize its Arg176-His177-Thr178-Arg179-Ser180 consensus sequence in FGF23, thus leading to FGF23 cleavage and separation of the N-terminal and C-terminal fragments. GalNT3, polypeptide N-acetylgalactosaminyltransferase 3; FAM20C, secretory protein kinase family with sequence similarity-20 member C; Arg, arginine; His, histidine; Thr, threonine; Ser, serine.