Abstract
Glycogen storage disease type IV (GSD IV, Andersen disease) is a rare autosomal recessive condition. The childhood neuromuscular subtype of GSD IV is characterised by a progressive skeletal myopathy with cardiomyopathy also reported in some individuals. We report a case of a 19-year-old man who presented with severe non-ischaemic dilated cardiomyopathy (NIDCM) necessitating heart transplantation, with biopsy showing aggregations of polyglucosan bodies in cardiac myocytes. He had no signs or symptoms of muscle weakness, liver dysfunction or neurologic involvement. A homozygous GBE1 c.607C>A (p.His203Asn) variant was identified. Our case is unusual in that our patient presented with an isolated NIDCM in the absence of other clinical manifestations of GSD IV. This case highlights the importance of considering storage disorders in young adults presenting with isolated NIDCM of unknown aetiology. It also emphasises the potential synergy between histopathological evaluation and genomic testing in enhancing diagnostic certainty.
Keywords: genetic screening / counselling, heart failure, pathology, genetics
Background
Glycogen storage disease type IV (GSD IV; MIM # 232500) is an autosomal recessive condition due to biallelic pathogenic variants in GBE1. Clinically, it can be classified into five subtypes with significant variability in severity, age of onset and clinical features.1 The phenotypic spectrum is wide and encompasses a fatal perinatal neuromuscular subtype which presents with fetal akinesia deformation sequence (FADS) and death in the neonatal period to a childhood neuromuscular subtype with symptom onset typically in the second decade of life. The childhood neuromuscular subtype of GSD IV has a variable course, with only a few cases having been previously reported.2 3 While some affected individuals with the childhood neuromuscular subtype have a mild skeletal myopathy, others can present with a more progressive myopathy and in some cases, dilated cardiomyopathy. There are rare reports of GSD IV patients presenting with primarily cardiac manifestations, but in those prior reports, those individuals also presented with skeletal muscle and or hepatic involvement.4–6
GSD IV is estimated to affect 1:600 000 to 1:800 000 individuals worldwide.7 This case highlights the importance of considering storage disorders in young adults presenting with isolated non-ischaemic dilated cardiomyopathy (NIDCM) of unknown aetiology. It also emphasises the synergistic role histopathological evaluation and genomic testing can play in enhancing diagnostic certainty.
Case presentation
A 19-year-old man presented with a 15-month history of progressively worsening shortness of breath, orthopnoea, exertional dyspnea and paroxysmal nocturnal dyspnea. He was found to have a left ventricular ejection fraction (LVEF) of 25% and was eventually diagnosed with NIDCM. The patient was not dysmorphic and his physical examination was otherwise normal including a normal neurological examination. His tone was normal as were his deep tendon reflexes. He had no muscle weakness or atrophy and there were no signs of primary liver disease. His medical history was only significant for crystal methamphetamine abuse, although he denied using it in the 2-year period prior to this presentation. After his LVEF depreciated to 10%–15%, an implantable cardioverter defibrillator was implanted and a left ventricular assist device placed as a bridge to eventual orthotopic heart transplantation which took place 5 months after his initial presentation.
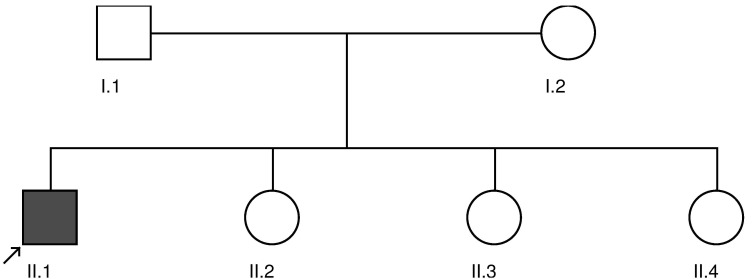
His family history was not notable for heart disease, myopathy, hepatic dysfunction, developmental delays, neurodegenerative disease or any known genetic conditions (figure 1). The proband’s parents were from the same town in Mexico, but consanguinity was denied.
Figure 1.
Proband marked with an arrow.
Investigations
Cardiac MRI demonstrated transmural delayed gadolinium enhancement in the lateral, anterolateral, and inferolateral walls from the apex to the base as well as within the apical inferior wall. Additionally, patchy midmyocardial enhancement was seen in the septum at the mid chamber. During his cardiac evaluation, right heart catheterisation and cardiac biopsy were performed and exhibited myocyte hypertrophy, interstitial fibrosis and periodic acid-Schiff (PAS)-positive and partially diastase-resistant intrasarcoplasmic inclusions. These findings were suggestive of a GSD.
Acid alpha-glucosidase enzyme activity was assessed to rule out Pompe disease and was within the normal range. Comprehensive inherited cardiomyopathy genetic testing which consisted of sequencing and concurrent deletion/duplication testing was sent. A total of 91 genes relevant to cardiomyopathy were tested, including LAMP2 (Danon disease) and PRKAG2. This genetic testing was non-diagnostic.
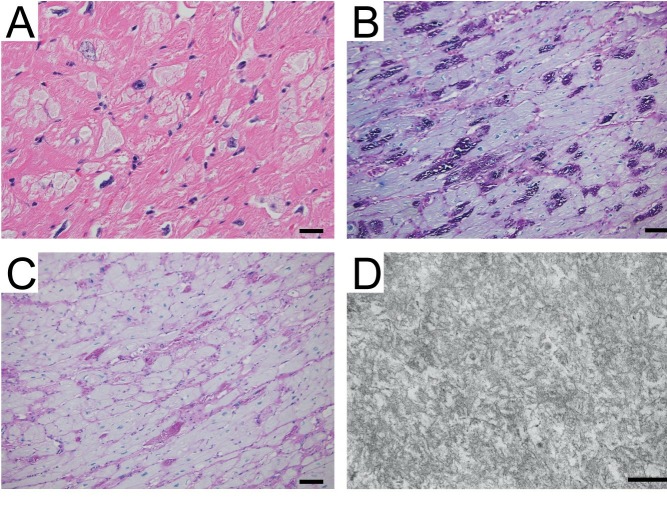
Given the negative cardiomyopathy gene panel, the cardiac muscle histopathology was re-evaluated. Polyglucosan bodies were identified by electron microscopy (figure 2). Genetic testing for GSD IV, and polyglucosan body myopathy type 1 and 2 which are due to pathogenic variants in GBE1, RBCK1 and GYG1, respectively, was performed. A homozygous GBE1 c.607C>A (p.His203Asn) variant was identified. It is absent from large population allele frequency databases including GnomAD, ExAC, 1000 Genomes Project and ESP6500.8–10 Multiple in silico prediction tools (table 1) are in agreement that this missense variant is deleterious; it occurs at a conserved position across species, and is functionally significant.11
Figure 2.
Cardiomyopathy due to glycogen storage disease type IV. An H&E stained section (A) shows hypertrophic cardiomyocytes with numerous cytoplasmic inclusions that have very pale grey staining (bar=25 µm). A periodic acid-Schiff (PAS) stain shows that the cardiomyocyte inclusions are strongly PAS-positive (B) and mostly sensitive to diastase digestion (C), consistent with glycogen inclusions in the cardiomyocytes. Electron microscopy was performed on tissue retrieved from paraffin block (D), revealing that the inclusions contain electron dense fibrillar structures consistent with polyglucosan (scale bar=200 nm).
Table 1.
In silico prediction tools11
| Algorithm | Prediction |
| CADD | ‘Damaging’ |
| PolyPhen-2 | ‘Probably damaging’ |
| SIFT | ‘Damaging’ |
| PROVEAN | ‘Damaging’ |
| MutationTaster | ‘Disease causing’ |
Outcome and follow-up
The patient has done well following transplant and continues to be followed closely in a cardiac transplant clinic as an outpatient. While this patient has what appears to be a primarily cardiac presentation at this time, we cannot rule out the possibility of his later developing a skeletal myopathy, liver disease or other clinical features of GSD IV. Additionally, severe cardiomyopathy in the setting of Adult Polyglucosan Body Disease (APBD; MIM # 263570) has also been reported.12 APBD is also due to GBE1 pathogenic variants and typically characterised by an adult onset of progressive neurogenic bladder, gait difficulties, peripheral neuropathy and executive dysfunction.13 This patient will therefore be monitored routinely in the genetics clinic to assess the potential development of additional manifestations of disease.
Given his diagnosis, the patient will also undergo genetic counselling and discuss the potential utility of family-specific carrier testing for his at-risk family members. Although his siblings are not known to be affected, there is variability in the age of onset and in the phenotypic presentation of GSD IV even within the same family. Each of his three siblings has a 25% chance of being affected. Additionally, if he plans on having more children, carrier testing for potential partners is an option and could be relevant to future family planning.
Discussion
GBE1 (OMIM 607839) is located on the short arm of chromosome 3 and encodes 1,4-alpha-glucan-branching enzyme which is also known as the glycogen branching enzyme. This enzyme catalyses the generation of α−1,6-glucosidic branches from α−1,4-linked glucose chains, which increase the solubility of the glycogen polymer.14 In GSD IV, biallelic pathogenic variants in GBE1 lead to enzyme deficiency. There is a resultant accumulation and deposition in tissue of abnormal glycogen or ‘polyglucosan bodies’ which resemble the plant starch amylopectin.15 16 More than 50 pathogenic variants in GBE1 have been reported in the literature and by clinical labs.17 The majority are missense variants, although nonsense, splice site, and small insertions and deletions, have also been reported. The GBE1 c.607C>A (p.His203Asn) variant was previously identified in a patient being assessed for a GSD by a private lab and was designated as a variant of uncertain significance.18 Phenotypic details were not provided for that patient and it is not clear if that patient was compound heterozygous or homozygous for the variant. The GBE1 c.607C>A (p.His203Asn) variant occurs in exon 5, an exon in which pathogenic variants have been previously reported.19 20
There have been reports of adolescent and adult GSD IV patients presenting with primarily cardiac manifestations, but in those prior reports, those individuals also presented with skeletal muscle and/or hepatic involvement.4–6 Our case is unusual in that our patient presented with an isolated NIDCM in the absence of other clinical manifestations of GSD IV. There is neither a specific treatment for GSD IV at this time nor are there consensus clinical guidelines for ongoing surveillance. Potentially informative evaluations can include routine neurological assessment, screening echocardiograms, abdominal ultrasound and liver function tests. Frequency of evaluation is predicated on clinical status and disease progression. Methamphetamine-associated cardiomyopathy is a well-recognised entity.21 It is not clear at this time if his prior drug use hastened the clinical progression of his NIDCM given his underlying genetically mediated disease.
Patient’s perspective.
I am just happy to have some answers. It was helpful that the doctors were willing to explain things more than once, and in more than one way.
Learning points.
Storage disorders should be a consideration in patients presenting with cardiomyopathy even in the absence of overt skeletal muscle or hepatic involvement.
Histopathology can play a critical role in narrowing the differential diagnosis in rare disorders.
GBE1 should be included in cardiomyopathy gene panels.
Footnotes
Contributors: MKN-K performed chart review and literature searches, collected and analysed the data, and reported the work described in the article. SC evaluated the patient, performed chart review, literature searches, reviewed and edited the article. JM and KDS were the pathologists involved in this case, they performed chart review, literature searches, reviewed and edited the article.
Funding: This study was funded by National Institutes of Health (http://dx.doi.org/10.13039/100000002) and grant no: 5T32GM007454.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Magoulas PL, El-Hattab AW. Glycogen storage disease type IV : Adam MP, Ardinger HH, Pagon RA, GeneReviews®. Seattle (WA): University of Washington, 2013:1993–2018. [PubMed] [Google Scholar]
- 2. Reusche E, Aksu F, Goebel HH, et al. . A mild juvenile variant of type IV glycogenosis. Brain Dev 1992;14:36–43. 10.1016/S0387-7604(12)80277-4 [DOI] [PubMed] [Google Scholar]
- 3. Schröder JM, May R, Shin YS, et al. . Juvenile hereditary polyglucosan body disease with complete branching enzyme deficiency (type IV glycogenosis). Acta Neuropathol 1993;85:419–30. 10.1007/bf00334454 [DOI] [PubMed] [Google Scholar]
- 4. Aksu T, Colak A, Tufekcioglu O. Cardiac involvement in glycogen storage disease type IV: two cases and the two ends of a spectrum. Case Rep Med 2012;2012:1–4. 10.1155/2012/764286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nase S, Kunze KP, Sigmund M, et al. . A new variant of type IV glycogenosis with primary cardiac manifestation and complete branching enzyme deficiency. In vivo detection by heart muscle biopsy. Eur Heart J 1995;16:1698-704 10.1093/oxfordjournals.eurheartj.a060797 [DOI] [PubMed] [Google Scholar]
- 6. Ewert R, Gulijew A, Wensel R, et al. . [Glycogenosis type IV as a seldom cause of cardiomyopathy - report about a successful heart transplantation]. Z Kardiol 1999;88:850–6. [DOI] [PubMed] [Google Scholar]
- 7. Chen Y, et al. . Glycogen storage disease : Scriver CR, Beaudet AS, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, The metabolic and molecular basis of inherited disease. 8 ed New York, NY: McGraw-Hill, 2001:1521–51. [Google Scholar]
- 8. Lek M, Karczewski KJ, Minikel EV, et al. . Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016;536:285–91. 10.1038/nature19057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Auton A, Brooks LD, Durbin RM, et al. . A global reference for human genetic variation. Nature 2015;526:68–74. 10.1038/nature15393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Server EV. NHLBI GO Exome Sequencing Project (ESP), Seattle, WA. 2018. http://evs.gs.washington.edu/EVS/
- 11. Ghosh R, Oak N, Plon SE. Evaluation of in silico algorithms for use with ACMG/AMP clinical variant interpretation guidelines. Genome Biol 2017;18:225 10.1186/s13059-017-1353-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Postler E, Sindern E, Vorgerd M, et al. . Fatal cardiomyopathy in adult in polyglucosan body disease]. Pathologe 2002;23:229–34. [DOI] [PubMed] [Google Scholar]
- 13. Klein CJ : Adam MP, Ardinger HH, Pagon RA, Adult Polyglucosan Body Disease GeneReviews®. Seattle (WA: University of Washington, Seattle, 2009:1993–2018. [PubMed] [Google Scholar]
- 14. Froese DS, Michaeli A, McCorvie TJ, et al. . Structural basis of glycogen branching enzyme deficiency and pharmacologic rescue by rational peptide design. Hum Mol Genet 2015;24:5667–76. 10.1093/hmg/ddv280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thon VJ, Khalil M, Cannon JF. Isolation of human glycogen branching enzyme cDNAs by screening complementation in yeast. J Biol Chem 1993;268:7509–13. [PubMed] [Google Scholar]
- 16. Moses S, Parvari R. The variable presentations of glycogen storage disease type IV: a review of clinical, enzymatic and molecular studies. Curr Mol Med 2002;2:177–88. 10.2174/1566524024605815 [DOI] [PubMed] [Google Scholar]
- 17. Stenson PD, Ball EV, Mort M, et al. . Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat 2003;21:577–81. 10.1002/humu.10212 [DOI] [PubMed] [Google Scholar]
- 18. Li SC, Chen CM, Goldstein JL, et al. . Glycogen storage disease type IV: novel mutations and molecular characterization of a heterogeneous disorder. J Inherit Metab Dis 2010;33(Suppl 3):S83–90. 10.1007/s10545-009-9026-5 [DOI] [PubMed] [Google Scholar]
- 19. Fernandez C, Halbert C, De Paula AM, et al. . Non-lethal neonatal neuromuscular variant of glycogenosis type IV with novel GBE1 mutations. Muscle Nerve 2010;41:269–71. 10.1002/mus.21499 [DOI] [PubMed] [Google Scholar]
- 20. Bao Y, Kishnani P, Wu JY, et al. . Hepatic and neuromuscular forms of glycogen storage disease type IV caused by mutations in the same glycogen-branching enzyme gene. J Clin Invest 1996;97:941–8. 10.1172/JCI118517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Won S, Hong RA, Shohet RV, et al. . Methamphetamine-Associated Cardiomyopathy. Clin Cardiol 2013;36:737–42. 10.1002/clc.22195 [DOI] [PMC free article] [PubMed] [Google Scholar]