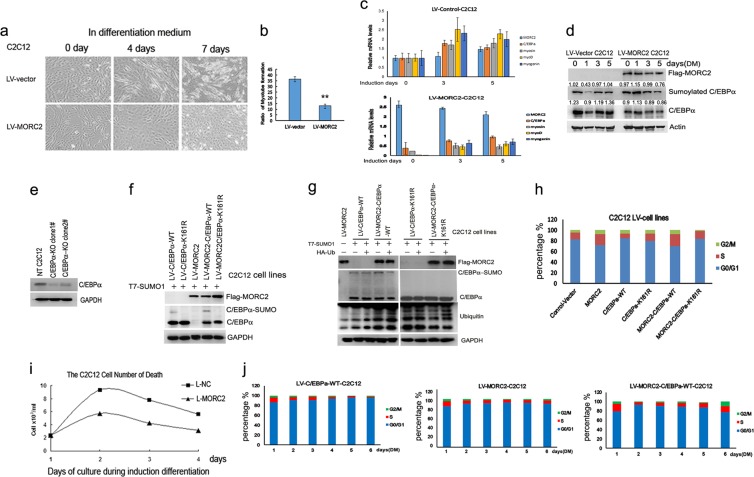
Fig. 4.
MORC2 regulates C/EBPα-mediated C2C12 muscle cell differentiation. a In differentiation medium MORC2 overexpressing C2C12 cells keep proliferating and do not start differentiation. Phase-contrast microphoto of C2C12 and LV-MORC2 differentiation cells at 0, 4, and 7 days of differentiation. Original magnification 10×. To induce skeletal muscle differentiation, when reaching 70–80% confluence, cells of infection with Lentivirus Flag-control vector (LV-NC) and Lentivirus Flag-MORC2 (LV-MORC2) were cultured in DM. DM was changed every 2 days and differentiation was completed in 7 days. b The ratios of myotube formation in LV-MORC2 cells (13.0% ± 1.5%) on day 6 of differentiation showed significant less than LV-control vector cells (36.4% ± 2.2%) P < 0.0001. c With the process from C2C12 myoblast to mature myocytes triggered by 2% horse serum, muscle-related protein molecules expression changed prior to simulation. The differentiating C2C12 myoblast cells was investigated by subjecting confluent C2C12 cells from GM (growth medium) to DM (differentiation medium) to induce differentiation for 7 days, and isolating mRNA extracts at the indicated time point. Real-time PCR detected the mRNA levels of myoD, myogenin, myosin and C/EBPα. The mRNA levels of MyoD (myogenic differentiation antigen), myogenin and myosin increased gradually in LV-control C2C12 cells (“3 group” and “5 group” compared to “0” group in upper panel) compared to “0 day” of differentiation (the mRNA levels of LV-control C2C12 in “0 day” of the differentiation were set to 1 and used as a control), but their expression in LV-MORC2 C2C12 cells showed no significant difference (“3 group” and “5 group” compared to “0” group in lower panel) compared to “0 day” (the mRNA levels of LV-MORC2 C2C12 were the relative ratio to the control vector in “0 day” of the differentiation, “0” group in lower panel). C/EBPα mRNA was decreased in the MORC2 overexpressing C2C12 compared to control C2C12 cells in “0 day” (Fig. 4c, lower panel compared to upper panel of “0 group”), but the C/EBPα mRNA expressed approximately equivalent levels in “3 days” and “5 days” compared to “0 day” (Fig. 4c, lower panel). d The expression levels of C/EBPα and MORC were detected with LV-MORC2 and control vector C2C12 cells during induction differentiation by western blotting. The differentiating C2C12 myoblast cells was investigated by subjecting confluent C2C12 cells to induce differentiation for 6 days, and isolating protein extracts at the indicated time point. “0 day” shown cells were cultured with growth medium. Total lysate from C2C12 cells undergoing differentiation was prepared on the days indicated and analyzed by immunoblotting with anti-MORC2, anti-C/EBPα and anti-GAPDH. The C/EBPα protein and its sumoylation levels are gradually increased in LV-control vector C2C12 cells (left panel, “5 days” and “3 days” compared to “1 day”), its protein and sumoylation levels were gradually decreased in LV-MORC2-C2C12 cells (right panel, “5 days” and “3 days” compared to “1 day”). e The C/EBPα-KO C2C12 cells were identified the knockout endogenous C/EBPα expression by western blot. C2C12 cells were transfected with the human C/EBPα double nickase plasmid (from Santa Cruz Company) and Selected with puromycin (2 g/mL) to product stable expressing C/EBPα-KO C2C12 cells. f MORC2 overexpression promotes the sumoylation of wild-type C/EBPα not mutant K161R in stable co-expressing C2C12 cells. These stable expressing of C2C12 cell lines including LV-control vector, LV-MORC2, LV-C/EBPα-WT, LV-C/EBPα-K161R, LV-MORC2-C/EBPα-WT and LV-MORC2-C/EBPα-K161R were transfected into T7-sumo1, western blot detected the sumoylation levels of C/EBPα with C/EBPα antibody. g MORC2 promotes the sumoylation of C/EBPα and its subsequent degradation. These stable expressing of C2C12 cell lines as showed in Fig. 4g were transfected into T7-sumo1 and HA-ub, western blot detected the sumoylation level of C/EBPα and its degradation. h MORC2 promotes C/EBPα-mediated C2C12 cell cycle transition from G1 to S. Flow cytometry analyzed the cell cycle transition with these stable expressing of C2C12 cell lines including LV-control vector, LV-MORC2, LV-C/EBPα-WT LV-C/EBPα-K161R, LV-MORC2-C/EBPα-WT and LV-MORC2-C/EBPα-K161R. i The died cell number of LV-NC and LV-MORC2 cells, cell viability was determined by Trypan blue dye exclusion assay. After differentiation induction, we counted dead cell number—collecting cell medium after 1 up to 4 days of differentiation—and expressed it as a percentage of dead cells, floating in medium, on total cells (sum of dead and viable cells, in suspension and adhering to plate). j MORC2 inhibits C/EBPα-mediated C2C12 cell differentiation via maintaining cell cycle progression. Flow cytometry analyzed cell cycle of LV-C/EBPα-WT, LV-MORC2 and LV-MORC2-C/EBPα-WT cells with the increasing differentiation induction days