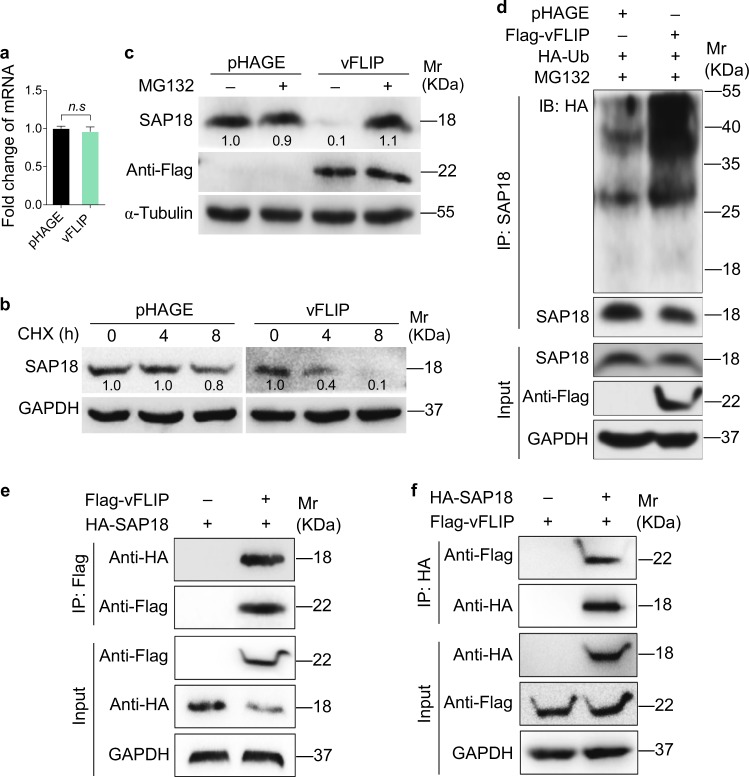
Fig. 3.
vFLIP degrades SAP18 through ubiquitin–proteasome pathway. a RT-qPCR was performed to examine the mRNA level of SA18 in HUVECs transduced with lentiviral vFLIP and its control pHAGE. Data are shown as mean ± s.d. (n.s., not significant). b Lentiviral pHAGE- and vFLIP-transduced HUVECs were treated with CHX (20 μg/ml) for 0 h, 4 h, and 8 h. Cells were lysed and then subjected to western blotting with indicated antibodies to monitor the stability of SAP18 protein. c Lentiviral pHAGE- and vFLIP-transduced HUVECs were treated with MG132 (20 μM) for 6 h and then subjected to western blotting with indicated antibodies to verify the SAP18 degradation pathway. d Lentiviral pHAGE- and vFLIP-transduced HUVECs were transfected with the HA-Ub construct, and then treated with MG132 (20 μM) for 6 h. Cells were subjected to anti-SAP18 immunoprecipitation assay (IP) for detection of SAP18 ubiquitination. e HUVECs were transfected with Flag-vFLIP construct alone, or co-transfected with HA-SAP18 construct. The interaction between vFLIP and SAP18 proteins was examined by immunoprecipitation with anti-Flag antibody. f HUVECs were transfected with HA-SAP18 construct alone, or co-transfected with Flag-vFLIP construct. The interaction between vFLIP and SAP18 proteins was examined by immunoprecipitation with anti-HA antibody