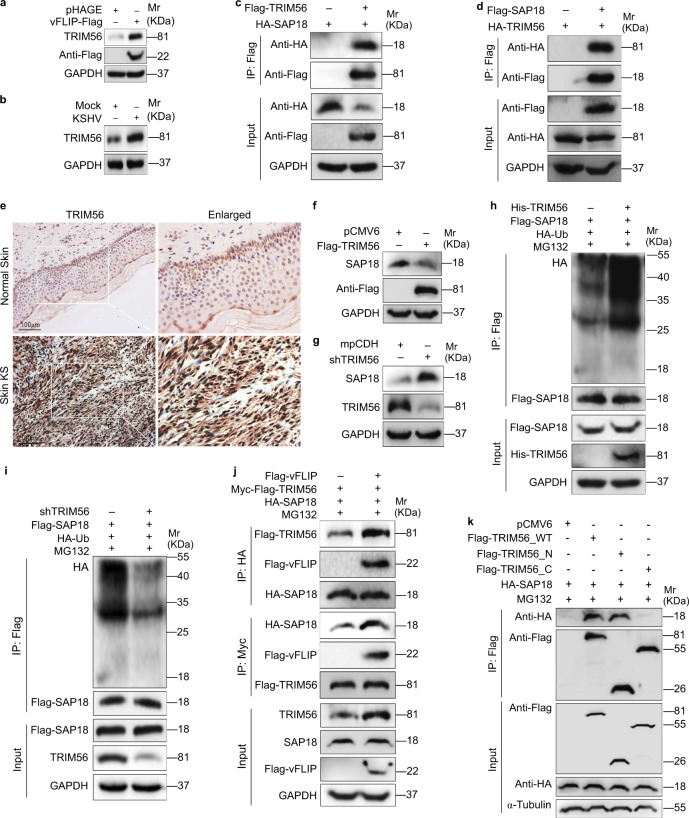
Fig. 4.
vFLIP increases and recruits TRIM56 to interact with and degrade SAP18. a Western blotting analysis of TRIM56 in HUVECs transduced with lentiviral vFLIP and its control pHAGE. b Western blotting analysis of TRIM56 in HUVECs infected with KSHV and its control PBS (Mock). c HUVECs were transfected with HA-SAP18 construct alone, or co-transfected with Flag-TRIM56 construct. The interaction between TRIM56 and SAP18 proteins was examined by immunoprecipitating with anti-Flag antibody. d HUVECs were transfected with HA-TRIM56 construct alone, or co-transfected with Flag-SAP18. The interaction between TRIM56 and SAP18 proteins was examined by immunoprecipitating with anti-Flag antibody. e The expression levels of TRIM56 in KS lesions and normal tissues were examined by immunohistochemistry assay (×200). f Western blotting analysis of SAP18 in HUVECs transfected with TRIM56 or its control pCMV6. g Western blotting analysis of SAP18 in HUVECs transduced with TRIM56 shRNA pool or its control mpCDH. h The HA-Ub and Flag-SAP18 plasmids were co-transfected with or without His-TRIM56 into HUVECs. The ubiquitinated SAP18 was detected by immunoprecipitating with anti-Flag antibody. i The HA-Ub and Flag-SAP18 plasmids were co-transduced with or without TRIM56 shRNA pool in HUVECs. The ubiquitinated SAP18 was detected by immunoprecipitating with anti-Flag antibody. j Immunoprecipitation analysis was adopted to examine the effect of vFLIP on the interaction between TRIM56 and SAP18 proteins. Cells were incubated with 20 μM MG132 for 6 h before immunoprecipitating the taq of TRIM56 or SAP18. k TRIM56_N and TRIM56_C were generated and used to examine the TRIM56-binding region with SAP18 by immunoprecipitation