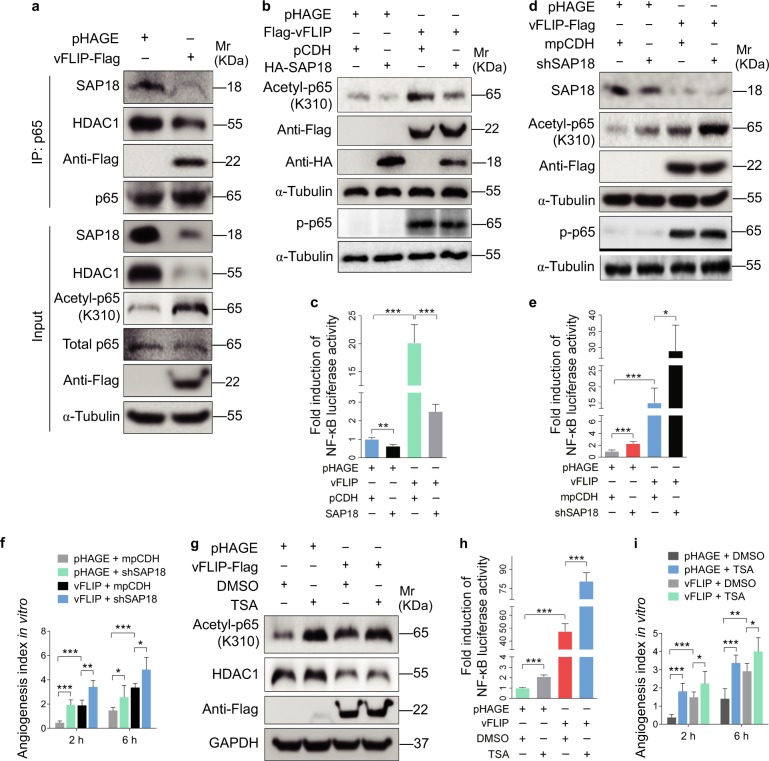
Fig. 6.
vFLIP decreases SAP18 and HDAC1 complex to promote cell motility and angiogenesis by enhancing acetylation of p65 subunit and NF-κB activation. a The interaction between p65 subunit and SAP18, or p65 subunit and HDAC1 was examined by immunoprecipitating with anti-p65 antibody in vFLIP and pHAGE-transduced HUVECs. Western blotting was adopted to examine SAP18, HDAC1, and acetylated p65 with indicated antibodies. b Western blotting analysis of acetylated and phosphorylated p65 in lentiviral vFLIP (Flag-vFLIP)- or its control pHAGE-infected HUVECs, followed by transduction with lentiviral SAP18 (HA-SAP18) or its control pCDH (pCDH), respectively, with the indicated antibodies. c NF-κB activity was assessed by using NF-κB reporter luciferase plasmid in HUVECs transfected with pHAGE or vFLIP plasmid, together with pCDH or SAP18 plasmid. Data are shown as mean ± s.d. (**P < 0.01 and ***P < 0.001). d Lentiviral vFLIP-transduced HUVECs were transduced with lentivirus expressing a mixture of shRNA1 and shRNA2 that target SAP18. Cells were subjected to western blotting for examination of acetylated and phosphorylated p65, SAP18. e NF-κB activity was assessed by using NF-κB reporter luciferase plasmid in HUVECs treated as in (d). Data are shown as mean ± s.d. (*P < 0.05 and ***P < 0.001). f Microtubule-formation assay was adopted in cells treated as in (d). Data are represented as mean ± s.d. (*P < 0.05, **P < 0.01, and ***P < 0.001). g Western blotting analysis of acetylated p65 level in lentiviral vFLIP-transduced HUVECs treated with HDAC inhibitor TSA or DMSO. h NF-κB activity was measured in cells as treated in (g). Data are shown as mean ± s.d. (***P < 0.001). i The in vitro angiogenesis capability was examined by microtubule-formation assay in cells treated as in (g). Data are represented as mean ± s.d. (*P < 0.05, **P < 0.01, and ***P < 0.001)