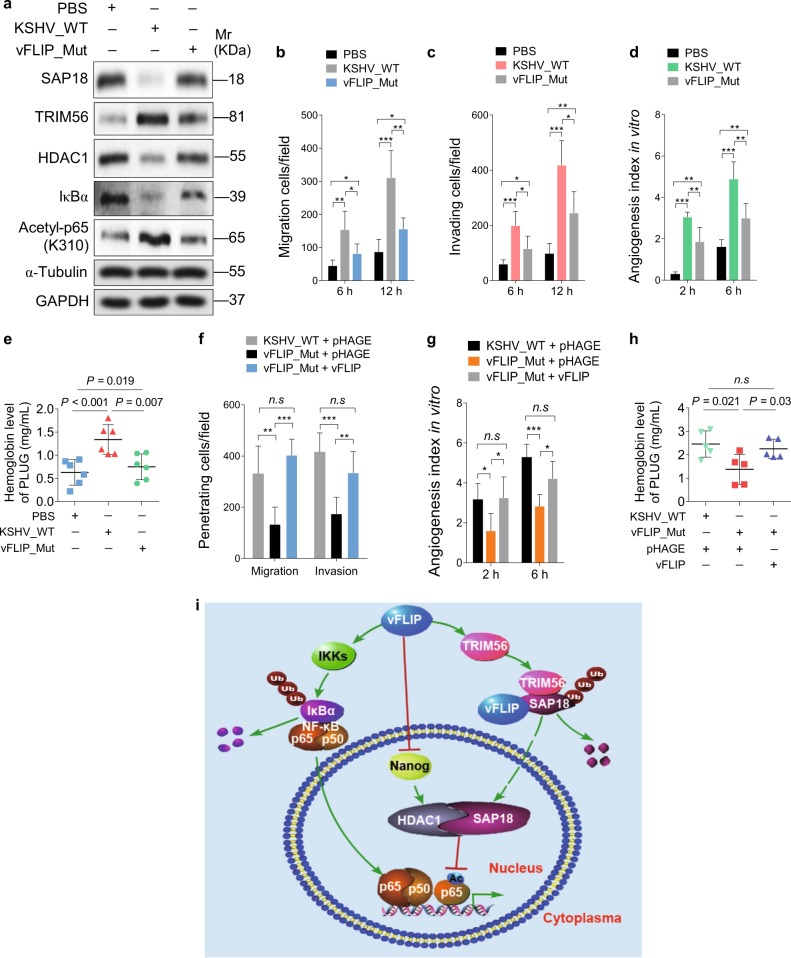
Fig. 7.
Lack of vFLIP restrains cell invasion and angiogenesis induced by KSHV. a Western blotting was performed with indicated antibodies to detect SAP18, TRIM56, HDAC1, IκBα, and acetylated p65 HUVECs treated with PBS (PBS), infected with wild-type KSHV (KSHV_WT) or vFLIP mutant virus (vFLIP-Mut). b Cells treated as in (a) were subjected to transwell migration assay. Mean ± s.d. is adopted to quantify the results (*P < 0.05, **P < 0.01, and ***P < 0.001). c Cells treated as in (a) were subjected to Matrigel invasion assay. Mean ± s.d. is adopted to quantify the results (*P < 0.05, **P < 0.01, and ***P < 0.001). d Cells treated as in (a) were subjected to microtubule-formation assay. Mean ± s.d. is adopted to quantify the results. (**P < 0.01 and ***P < 0.001). e Cells treated as in (a) were adopted to Matrigel plug assay in mice. The level of hemoglobin in plug tissues treated was measured by comparing the standard curve. Mean ± s.d. is adopted to quantify the results, with six tumors for each group. f Transwell migration and Matrigel invasion assays were performed in HUVECs infected with wild-type KSHV or vFLIP mutant virus, followed by transduction of lentiviral vFLIP at 12 h post-seeding. Mean ± s.d. is adopted to quantify the results (n.s., not significant, **P < 0.01 and ***P < 0.001). g Cells treated as in (f) were subjected to microtubule-formation assay. Mean ± s.d. is adopted to quantify the results (n.s., not significant, *P < 0.05 and ***P < 0.001). h Cells treated as in (f) were adopted to Matrigel plug assay in mice. The level of hemoglobin in plug tissues treated in (f) was measured by comparing the standard curve. Mean ± s.d. is adopted to quantify the results, with five tumors for each group. i Schematic illustration for the mechanism of vFLIP-induced endothelial cell motility and angiogenesis. During KSHV latent infection, vFLIP recruits E3 ubiquitin ligase TRIM56, leading to the ubiquitination and degradation of SAP18. Meanwhile, vFLIP inhibits HDAC1 promoter by suppressing transcription factor Nanog, resulting in downregulation of HDAC1. The decreased SAP18/HDAC1 complex enhances acetylation of p65 subunit and activates the NF-κB signal pathway