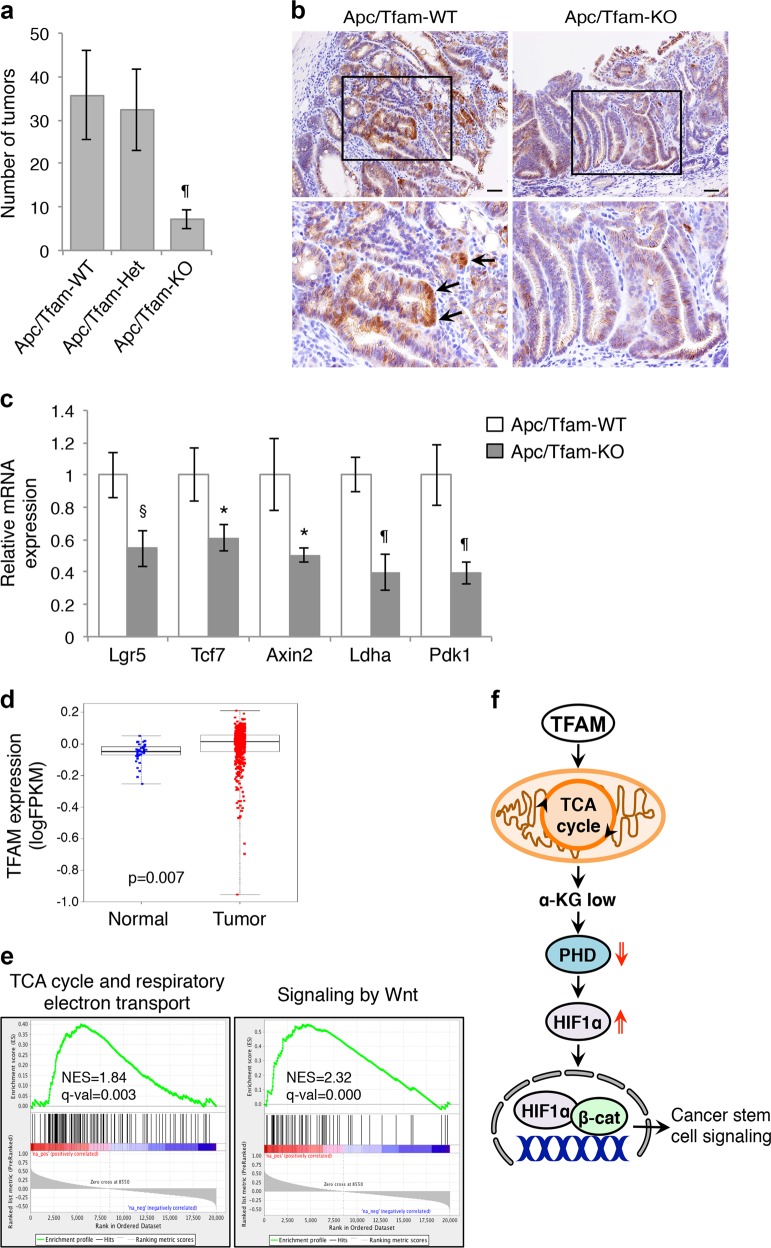
Fig. 7.
Knockout of TFAM inhibits tumorigenesis in Apc-mutant mice. a The number of intestinal adenomas was counted in the following three cohorts of mice at 6 months: Apcf/+/TFAM+/+/Villin-Cre (Apc/TFAM-WT, n = 12), Apcf/+/TFAMf/+/Villin-Cre (Apc/TFAM-Het, n = 12), and Apcf/+/TFAMf/f/Villin-Cre (Apc/TFAM-KO, n = 11). Data represent the mean ± SD (¶p < 0.0001 compared to the Apc/TFAM-WT group). b TFAM-loss reduced nuclear localization β-catenin as detected by IHC staining using the anti-β-catenin antibody. The boxed regions in upper images were enlarged and shown at the bottom, and arrows indicate nuclear β-catenin staining. Scale bar, 50 μm. c Knockout of TFAM decreased the expression of genes associated with cancer stem cells. The relative expression of target genes downstream of Wnt/β-catenin (including Lgr5, Tcf7, and Axin2) or HIF1α (including LdhA and Pdk1) was determined using qRT-PCR in tumors derived from Apc/TFAM-WT and Apc/TFAM-KO mice. Tumor tissues from three mice per group were analyzed. Data represent the mean ± SD (¶p < 0.0001, §p < 0.001, and *p < 0.01 compared to Apc/TFAM-WT). d TCGA CRC RNA-seq dataset was used to analyze the expression of TFAM. The expression of TFAM in tumor vs. normal samples was compared using linear mixed models (p = 0.007). e The GSEA was performed using the TCGA CRC RNA-seq dataset to identify gene sets that have positive correlations with TFAM expression. Enrichment plots showed significant correlation of the TCA cycle and respiratory electron transport (NES = 1.84, FDR q-val = 0.003) and the signaling by Wnt (NES = 2.32, FDR q-val = 0.000) pathway with TFAM expression in CRC patients. f Diagram showing TFAM-mediated regulation of mitochondrial retrograde signaling in colon cancer cells. Our study here demonstrates that TFAM is required to maintain the mitochondrial integrity and TCA cycle activity. When the mitochondrial function is maintained, the production of TCA metabolite α-KG and α-KG-dependent PHD2 activity are kept at lower levels, which allows HIF1α stabilization under hypoxia and activation of Wnt/β-catenin-mediated cancer stem cell signaling