Abstract
BACKGROUND
Universal antiretroviral therapy (ART) with annual population testing and a multidisease, patient-centered strategy could reduce new human immunodeficiency virus (HIV) infections and improve community health.
METHODS
We randomly assigned 32 rural communities in Uganda and Kenya to baseline HIV and multidisease testing and national guideline–restricted ART (control group) or to baseline testing plus annual testing, eligibility for universal ART, and patient-centered care (intervention group). The primary end point was the cumulative incidence of HIV infection at 3 years. Secondary end points included viral suppression, death, tuberculosis, hypertension control, and the change in the annual incidence of HIV infection (which was evaluated in the intervention group only).
RESULTS
A total of 150,395 persons were included in the analyses. Population-level viral suppression among 15,399 HIV-infected persons was 42% at baseline and was higher in the intervention group than in the control group at 3 years (79% vs. 68%; relative prevalence, 1.15; 95% confidence interval [CI], 1.11 to 1.20). The annual incidence of HIV infection in the intervention group decreased by 32% over 3 years (from 0.43 to 0.31 cases per 100 person-years; relative rate, 0.68; 95% CI, 0.56 to 0.84). However, the 3-year cumulative incidence (704 incident HIV infections) did not differ significantly between the intervention group and the control group (0.77% and 0.81%, respectively; relative risk, 0.95; 95% CI, 0.77 to 1.17). Among HIV-infected persons, the risk of death by year 3 was 3% in the intervention group and 4% in the control group (0.99 vs. 1.29 deaths per 100 person-years; relative risk, 0.77; 95% CI, 0.64 to 0.93). The risk of HIV-associated tuberculosis or death by year 3 among HIV-infected persons was 4% in the intervention group and 5% in the control group (1.19 vs. 1.50 events per 100 person-years; relative risk, 0.79; 95% CI, 0.67 to 0.94). At 3 years, 47% of adults with hypertension in the intervention group and 37% in the control group had hypertension control (relative prevalence, 1.26; 95% CI, 1.15 to 1.39).
CONCLUSIONS
Universal HIV treatment did not result in a significantly lower incidence of HIV infection than standard care, probably owing to the availability of comprehensive baseline HIV testing and the rapid expansion of ART eligibility in the control group. (Funded by the National Institutes of Health and others; SEARCH ClinicalTrials.gov number, .)
ANTIRETROVIRAL THERAPY (ART) HAS the dual benefit of improving the health of individual persons and of reducing transmission of human immunodeficiency virus (HIV) infection.1–3 With 1.8 million new HIV infections and 1.0 million deaths annually, it is clear that the full individual and public health gains from effective treatment of all HIV-infected persons have yet to be realized.4
New approaches to universal HIV treatment must overcome many barriers. An estimated 9.2 million of the 36.9 million persons with HIV do not know their HIV status.4 Persons who receive a diagnosis of HIV infection face health care systems that are unresponsive to life-stage and gender-specific needs, have discriminatory policies, and lack patient-centered care.5,6 As a result, only an estimated 47% of persons living with HIV have viral suppression — far short of the 73% target for 2020 set by the joint United Nations Program on HIV/AIDS (UNAIDS). The challenge now is finding HIV-infected persons who are not currently receiving care for HIV and tailoring approaches to engage and retain those persons in care. We postulated that in rural Uganda and Kenya, reaching these persons would require addressing their health priorities beyond HIV, at a place they desire to attend, and with care centered on their needs. We conducted a randomized trial to test the hypothesis that universal HIV treatment and annual testing delivered with a community-based, multidisease, patient-centered approach would result in a lower number of new HIV infections and better community health than the current standard of care.
METHODS
TRIAL DESIGN AND POPULATION
The Sustainable East Africa Research in Community Health (SEARCH) trial was a cluster-randomized trial that was conducted in three regions of rural Uganda and Kenya from 2013 through 2017. The randomization units were communities of 9000 to 11,000 persons that were defined by recent national census and administrative boundaries. A total of 54 communities in target regions were selected on the basis of criteria that included the presence of a government health clinic that provided ART and distance from other potential trial communities (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).
Communities were pair-matched on the basis of geographic region, population density, number of trading centers, variety of occupations, and mobility patterns. The best-matching 16 pairs were randomly assigned to baseline HIV testing and multidisease testing at health fairs and national guideline–restricted antiretroviral therapy (control group) or to baseline HIV and multidisease testing plus annual testing, eligibility for universal antiretroviral therapy, and patient-centered care (intervention group) (Fig. S2 in the Supplementary Appendix). Randomization was performed at a participatory public event attended by community leaders and members.7 The trial population described in this report included all residents 15 years of age or older. After the release of the 2015 World Health Organization ART guidelines in which universal ART was recommended, the data and safety monitoring board approved a modification to the protocol that reduced the duration of follow-up for evaluation of the primary end point from 5 years to 3 years.8
CENSUS
At baseline, we enumerated and enrolled residents with the use of a household census in partnership with community leaders. Demographic information, household socioeconomic information, migration data, and coordinates of the geographic location were collected at these visits.9 In addition to residents’ names, biometric identifiers based on each resident’s digital fingerprint were used to identify residents during their participation in testing and care activities in the community.
COMMUNITY HEALTH CAMPAIGNS
After completing the baseline census, we conducted mobile, 2-week, multidisease health campaigns under large tents in all communities during weekdays, evenings, and weekends in collaboration with local health units and the Ministry of Health in Uganda and in Kenya; for persons who did not attend the campaign, testing was performed at their home or other location of their choice.9 Persons who were found to have HIV infection, diabetes, or hypertension received counseling and clinic appointments. HIV-infected persons also had their CD4+ T-cell count and HIV RNA levels measured and received a one-time round-trip transportation voucher for the first clinic visit.9,10 Repeat campaigns including HIV testing were conducted annually in the intervention communities only. After 3 years, campaigns were conducted in all communities for assessment of the trial end points.
ANTIRETROVIRAL THERAPY AND TREATMENT OF NONCOMMUNICABLE DISEASE
The SEARCH treatment intervention was designed to remove patient-level barriers and maximize the efficiency of the health care system. For HIV-infected persons who were not receiving ART, appointments to initiate or restart ART were made as soon as possible, within 7 days at most, after HIV testing. To facilitate linkage to care, the clinic staff introduced themselves in person or by mobile phone, provided persons with a hotline that they could contact by telephone or text message if they had questions or needed support, and called or sent text messages for reminders about clinic visits. We provided patient-centered care for HIV infection, diabetes, and hypertension at government clinics that were augmented by trial staff.11 Key components of care included 3-month visit intervals, flexible hours, reduced wait time at clinics, and a welcoming staff. We offered ART to all persons with HIV infection. For persons who were assessed as ineligible to receive treatment on the basis of national guidelines, the trial provided efavirenz in combination with tenofovir–emtricitabine (Truvada), which was donated by Gilead Sciences. Gilead had no role in the design of the trial, in the collection or analysis of the data, or in the preparation of the manuscript, although an industry representative from Gilead was a member of the protocol team and scientific advisory board. For all persons who had hypertension or diabetes, care followed standard algorithms (Fig. S3 in the Supplementary Appendix).
At clinics in control communities, ART, hypertension therapy, and diabetes therapy were provided in accordance with national guidelines. To mitigate staffing shortages that could slow implementation of updated ART guidelines, we provided additional staff at control clinics. Control clinics implemented ART guidelines that were specific to Uganda and Kenya; during the trial, the threshold for eligibility for ART in these countries expanded from a specific CD4+ T-cell count (which had ranged from ≤350 cells per cubic millimeter to <500 cells per cubic millimeter) to universal treatment (regardless of CD4+ T-cell count) (Fig. S4 in the Supplementary Appendix).
END POINTS
The primary end point of the trial was the cumulative incidence of HIV infection (confirmed by the Bio-Rad Geenius HIV 1/2 Confirmatory Assay and Western blot testing) at 3 years among residents enumerated in the baseline census (Table S1 in the Supplementary Appendix). Secondary end points associated with HIV infection included the interim annual incidence of HIV infection in the intervention group, the time to initiation of ART, and viral suppression (defined as HIV RNA <500 copies per milliliter). Secondary health end points included death, incident tuberculosis or death due to illness, and control of hypertension and diabetes among the HIV-infected persons and in the overall population.
TRIAL OVERSIGHT
The trial protocol was approved by the ethics committees at the University of California, San Francisco; the Kenya Medical Research Institute; and Makerere University School of Medicine in Uganda. Community-level consent (described in the protocol) and oral informed consent from individual participants were provided for the census enumeration and the health campaigns, and written informed consent was provided in cases in which a participant was ineligible for ART on the basis of country guidelines. The trial was conducted in accordance with the principles of the Declaration of Helsinki, with oversight by the data and safety monitoring board. The trial protocol and statistical analysis plan are available at NEJM.org. The authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.
STATISTICAL ANALYSIS
We estimated that the sample size of 32 communities would provide 80% power to detect a cumulative incidence of HIV infection that was 40% lower in the intervention group than in the control group under conservative assumptions (i.e., a cumulative incidence of 1% in the control group, a matched-pair coefficient of variation of 0.4, and ≥2700 HIV-negative persons with outcomes measured in each community). Under less conservative assumptions, including model-based simulations, the trial was powered to detect a cumulative incidence that was 25% lower in the intervention group than in the control group.
We compared the cumulative incidence of HIV infection in the two trial groups using a two-stage approach. First, we calculated the percentage of HIV-negative residents who had seroconversion in each community. Next, we compared these percentages in the trial groups using community-level targeted maximum likelihood estimation; we selected adjustment variables (candidates included baseline prevalence of HIV infection and male circumcision coverage) using cross-validation.12 Statistical inference was based on Student’s t-distribution with 15 degrees of freedom and accounted for the pair-matched design.
We used an analogous approach to compare the secondary end points in the two groups. Viral suppression, control of hypertension, and control of diabetes were estimated for each community; differences in characteristics between persons with measured outcomes and persons with missing outcomes were adjusted with the use of individual-level targeted maximum likelihood estimation. The probability of ART initiation, death, and HIV-associated tuberculosis or death were estimated in each community by the Kaplan–Meier method. Estimates of HIV care cascade coverage (i.e., the percentage of HIV-infected persons who knew their status, the percentage of those who knew their status who had received ART, the percentage of those who had received ART who had HIV viral suppression, and the percentage of all HIV-infected persons with viral suppression) included data from persons who migrated into the community (and were therefore not residents in the community at the time of baseline testing) and adjusted for missing data. Annual incidence rates of HIV infection in communities in the intervention group were compared over time with the use of Poisson generalized estimating equations with an exchangeable covariance matrix.
RESULTS
TRIAL POPULATION
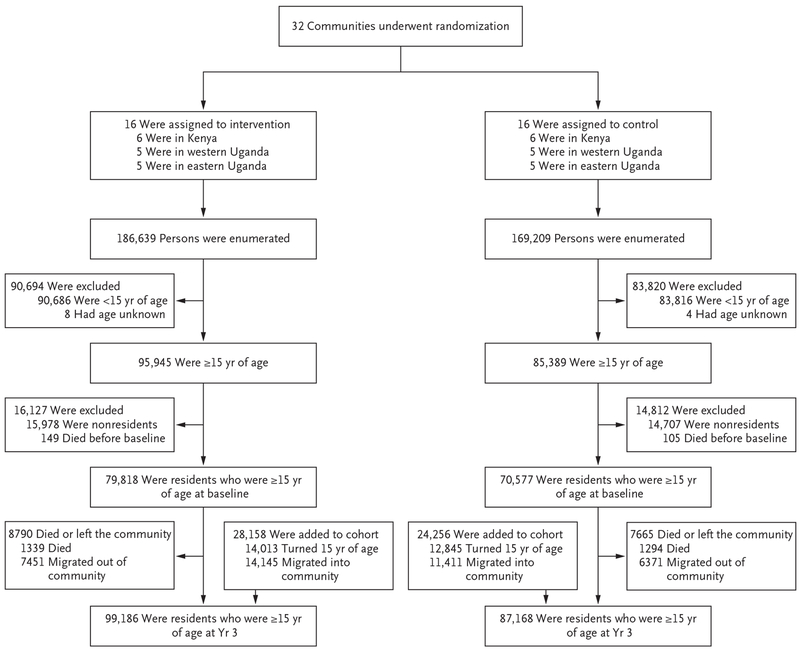
We enumerated 355,848 persons in the baseline census, of whom 150,395 were residents 15 years of age or older (Fig. 1). Of these residents, 45% were male and 96% were stable residents (defined as residents who had spent at least 6 months of the previous year in the trial community) (Table 1). The demographic characteristics of the two trial groups were balanced at baseline (Table S2 in the Supplementary Appendix). Male circumcision coverage varied across regions: 14% of the men in western Uganda, 40% in eastern Uganda, and 46% in Kenya reported having been circumcised (Fig. S5 in the Supplementary Appendix). Among 135,484 persons whose HIV serostatus was known, the baseline prevalence of HIV infection was 19% in Kenya, 7% in western Uganda, and 4% in eastern Uganda (Fig. S6 in the Supplementary Appendix). Among 13,529 persons known to be HIV-infected at baseline, 52% had a CD4+ T-cell count of more than 500 per cubic millimeter.
Figure 1.
Trial Cohort.
Table 1.
Characteristics of the Residents at Baseline, According to Geographic Region.✲
| Characteristic | Western Uganda (N = 47,328) | Eastern Uganda (N = 49,195) | Kenya (N = 53,872) | Total (N = 150,395) |
|---|---|---|---|---|
| no. of residents/total no. (%) | ||||
| Male sex | 21,325/47,328 (45.1) | 22,633/49,195 (46.0) | 24,023/53,872 (44.6) | 67,981/150,395 (45.2) |
| Age category | ||||
| 15–20 yr | 10,768/47,328 (22.8) | 13,282/49,195 (27.0) | 12,605/53,872 (23.4) | 36,655/150,395 (24.4) |
| 21–49 yr | 27,480/47,328 (58.1) | 26,636/49,195 (54.1) | 29,906/53,872 (55.5) | 84,022/150,395 (55.9) |
| ≥50 yr | 9,080/47,328 (19.2) | 9,277/49,195 (18.9) | 11,361/53,872 (21.1) | 29,718/150,395 (19.8) |
| Marital status | ||||
| Single | 13,918/47,256 (29.5) | 14,273/49,027 (29.1) | 14,893/53,757 (27.7) | 43,084/150,040 (28.7) |
| Married, including polygamous marriage | 27,465/47,256 (58.1) | 29,209/49,027 (59.6) | 32,512/53,757 (60.5) | 89,186/150,040 (59.4) |
| Widowed, divorced, or separated | 5,873/47,256 (12.4) | 5,545/49,027 (11.3) | 6,352/53,757 (11.8) | 17,770/150,040 (11.8) |
| Polygamous marriage | 3,393/47,249 (7.2) | 7,199/49,022 (14.7) | 8,193/53,753 (15.2) | 18,785/150,024 (12.5) |
| Educational level | ||||
| Below primary school | 26,514/47,299 (56.1) | 30,041/49,098 (61.2) | 40,048/53,663 (74.6) | 96,603/150,060 (64.4) |
| Completed primary school | 8,223/47,299 (17.4) | 6,328/49,098 (12.9) | 7,232/53,663 (13.5) | 21,783/150,060 (14.5) |
| Any secondary school or higher | 12,562/47,299 (26.6) | 12,729/49,098 (25.9) | 6,383/53,663 (11.9) | 31,674/150,060 (21.1) |
| Occupation† | ||||
| Formal sector | 10,383/47,247 (22.0) | 12,026/49,022 (24.5) | 13,077/53,762 (24.3) | 35,486/150,031 (23.7) |
| High-risk informal sector | 1,122/47,247 (2.4) | 689/49,022 (1.4) | 6,368/53,762 (11.8) | 8,179/150,031 (5.5) |
| Low-risk informal sector | 30,452/47,247 (64.5) | 32,682/49,022 (66.7) | 27,960/53,762 (52.0) | 91,094/150,031 (60.7) |
| Other | 3,141/47,247 (6.6) | 1,722/49,022 (3.5) | 1,933/53,762 (3.6) | 6,796/150,031 (4.5) |
| No job or disabled | 2,149/47,247 (4.5) | 1,903/49,022 (3.9) | 4,424/53,762 (8.2) | 8,476/150,031 (5.6) |
| Household wealth index quintile‡ | ||||
| First, indicating least wealth | 10,559/47,158 (22.4) | 8,040/49,080 (16.4) | 5,355/53,661 (10.0) | 23,954/149,899 (16.0) |
| Second | 10,734/47,158 (22.8) | 9,455/49,080 (19.3) | 5,937/53,661 (11.1) | 26,126/149,899 (17.4) |
| Third | 10,019/47,158 (21.2) | 10,388/49,080 (21.2) | 9,412/53,661 (17.5) | 29,819/149,899 (19.9) |
| Fourth | 8,595/47,158 (18.2) | 10,949/49,080 (22.3) | 13,543/53,661 (25.2) | 33,087/149,899 (22.1) |
| Fifth, indicating most wealth | 7,251/47,158 (15.4) | 10,248/49,080 (20.9) | 19,414/53,661 (36.2) | 36,913/149,899 (24.6) |
| Stable residents§ | 44,753/47,328 (94.6) | 47,148/49,195 (95.8) | 51,969/53,870 (96.5) | 143,870/150,393 (95.7) |
| Residents living with HIV | 2,873/43,769 (6.6) | 1,590/44,764 (3.6) | 9,066/46,951 (19.3) | 13,529/135,484 (10.0) |
| Residents with prevalent hypertension¶ | 3,951/19,877 (19.9) | 4,785/18,503 (25.9) | 3,128/17,381 (18.0) | 11,864/55,761 (21.3) |
HIV denotes human immunodeficiency virus.
A formal sector occupation was defined as a teacher, student, government worker, military worker, health worker, or factory worker. A high-risk informal sector occupation was defined as a fishmonger, fisher, bar owner, bar worker, transportation worker, or tourism worker. A low-risk informal sector occupation was defined as a farmer, shopkeeper, market vendor, hotel worker, homemaker, household worker, construction worker, or miner.
Quintiles were based on a principle-component analysis of the household wealth survey and were calculated at the level of the household.
Stable residents were defined as residents who had spent at least 6 months of the previous year in the trial community.
Adults 30 years of age or older were included in the analysis.
POPULATION-BASED HIV TESTING AND UPTAKE OF TREATMENT
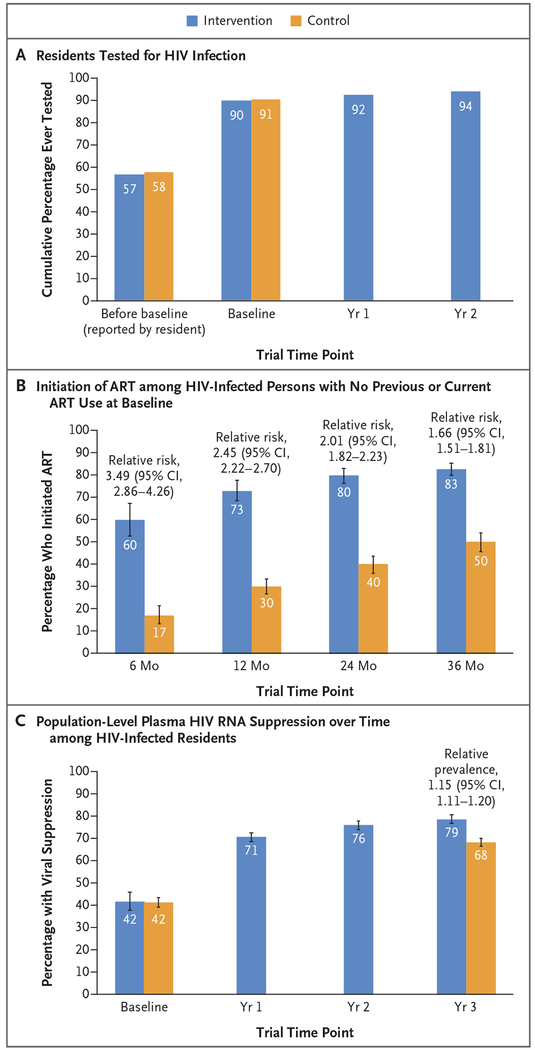
Before the start of baseline testing in the trial, approximately 57% of residents reported having undergone previous HIV testing (Fig. 2A). During baseline testing campaigns, we tested 90% of all census-enumerated residents in the intervention group and 91% in the control group. After the first-year health campaigns in the intervention group, 92% of residents, including those who migrated into the communities, had undergone HIV testing at least once; this percentage increased to 94% after the second year. By the 3-year end-point assessment, we achieved cumulative HIV testing coverage of 98% of residents in the intervention group and 96% of residents in the control group, including residents who migrated into each of the two groups of communities.
Figure 2. HIV Testing, Uptake of Treatment, and Viral Suppression.
Panel A shows the cumulative percentage of residents who underwent human immunodeficiency virus (HIV) testing before and during the trial. The values labeled as “before baseline” are the percentages of the 138,052 residents (72,978 in the intervention group and 65,074 in the control group) who reported at the time of baseline testing that they had undergone previous HIV testing. The remaining values in Panel A are the percentages of all residents at the time of annual testing (excluding persons who migrated out of the community and persons who died and including persons who were newly 15 years of age and persons who migrated into the community, identified through the second census that was conducted at year 3) who had at least one documented HIV test result. At baseline, the assessment included 79,818 residents in the intervention group and 70,577 residents in the control group; at year 1, the assessment included 89,994 residents in the intervention group, and at year 2, the assessment included 93,008 residents in the intervention group. Panel B shows the percentage of persons who initiated antiretroviral therapy (ART) among HIV-infected persons with no previous or current ART use at baseline. The assessment included 3002 residents in the intervention group and 2950 residents in the control group. Community-level estimates of the probability of initiating ART by 6, 12, 24, and 36 months were calculated by the Kaplan–Meier method; data from patients who died or who migrated out of the community were censored at the time of death or out-migration. The trial groups were compared with the use of community-level targeted maximum likelihood estimation. Panel C shows population-level plasma HIV RNA suppression over time among HIV-infected residents. The assessment included all residents at the time of annual testing (excluding persons who migrated out of the community and persons who died, but including residents who were newly 15 years of age and those who migrated into the community, identified through the second census that was conducted at year 3). HIV RNA suppression was assessed in 5347 residents in the intervention group and in 4192 residents in the control group at baseline; in 6269 residents in the intervention group at year 1; in 6348 residents in the intervention group at year 2; and in 6800 residents in the intervention group and 6051 residents in the control group at year 3. Community-level estimates of suppression were adjusted for incomplete measures of HIV serostatus and HIV RNA with the use of individual-level targeted maximum likelihood estimation (adjustment variables included sex, age group, marital status, educational level, occupation, alcohol use, household wealth, mobility, previous HIV testing, and care status). The estimated total number of HIV-positive persons was 15,399 at baseline and was 15,748 at year 3. The trial groups were compared with the use of community-level targeted maximum likelihood estimation. I bars in Panels B and C indicate 95% confidence intervals.
Among 5952 HIV-infected persons with no previous or current ART use, the percentage of persons who initiated ART was higher in the intervention group than in the control group after 6, 12, 24, and 36 months (Fig. 2B). Similar trends were seen across all baseline strata of CD4+ T-cell counts (Fig. S7 in the Supplementary Appendix), including a CD4+ T-cell count of less than 350 per cubic millimeter, which was the threshold for eligibility for ART that had been indicated for all persons at the start of the trial. In the intervention group, population-level HIV RNA suppression among all HIV-infected persons increased from 42% at baseline to 71% after 1 year (Fig. 2C). At 3 years, an estimated 92% of HIV-infected persons knew their status, 95% of these persons had received ART, and 90% of those who received ART had viral suppression. In contrast, in the control group at 3 years, 91% of HIV-infected persons knew their status, 86% of them had received ART, and 87% of those who received ART had viral suppression (Table S3 in the Supplementary Appendix).
At 3 years, among all HIV-infected persons, the prevalence of viral suppression was 15% higher in the intervention group than in the control group (79% vs. 68%; relative prevalence, 1.15; 95% confidence interval [CI], 1.11 to 1.20) (Fig. 2C). In the intervention group at 3 years, the prevalence of viral suppression was higher among women (81%) than among men, but 74% of men still had viral suppression; viral suppression among youth 15 to 24 years of age was 55%. Trends across subgroups were similar in the control group, although the prevalence of viral suppression was consistently lower in the control group than in the intervention group among both men and women and across all age strata and all geographic regions. Among persons who began the trial with viral suppression, 96% of those in the intervention group and 95% of those in the control group maintained viral suppression after 3 years. (Detailed data are provided in Figs. S8 through S10 in the Supplementary Appendix.)
INCIDENCE OF HIV INFECTION
Among the 117,114 adults in the HIV-incidence cohort (which comprised persons who were at least 15 years of age, were stable residents, and were HIV-negative at baseline), the primary end point was evaluated in 49,590 of 61,676 persons (80%) in the intervention group and in 45,493 of 55,438 persons (82%) in the control group (Figs. S11 and S12 in the Supplementary Appendix). A total of 704 confirmed new HIV infections were reported. The 3-year cumulative incidence of HIV infection in the intervention group was 0.77% (0.25 cases per 100 person-years) and was not significantly different from the cumulative incidence in the control group (0.81%; 0.27 cases per 100 person-years) (relative risk, 0.95; 95% CI, 0.77 to 1.17). Similarly, no significant differences in the 3-year cumulative incidence of HIV infection were observed either in prespecified subgroups (men, women, persons 15 to 24 years of age, persons older than 24 years, nonmobile persons, and uncircumcised men) or among geographic regions (Figs. S13 and S14 in the Supplementary Appendix). The annual incidence of HIV infection, which was evaluated in the intervention group only, declined over the course of the trial (Table 2). The annual incidence of HIV infection was estimated from cohorts of more than 50,000 adults who were tested annually (Fig. S15 in the Supplementary Appendix); the incidence at 3 years was 32% lower than that at 1 year (relative rate, 0.68; 95% CI, 0.56 to 0.84). Declines in the annual HIV incidence varied across regions, with a 46% reduction in Kenya, virtually no change in western Uganda, and a 39% reduction in eastern Uganda at 3 years. The decline in the incidence among men was more than double that among women (50% vs. 18%) (Fig. S16 in the Supplementary Appendix).
Table 2.
Change in the Annual Incidence of HIV Infection over Time in the Intervention Group.✲
| Region | Incidence Rate per 100 Person-Yr | Relative Rate (95% CI)† | ||
|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | ||
| All regions | 0.43 | 0.38 | 0.31 | 0.68 (0.56–0.84) |
| Kenya | 0.69 | 0.62 | 0.39 | 0.54 (0.39–0.75) |
| Western Uganda | 0.35 | 0.35 | 0.38 | 1.03 (0.73–1.44) |
| Eastern Uganda | 0.29 | 0.19 | 0.18 | 0.61 (0.39–0.95) |
The annual incidence rate of HIV infection per 100 person-years was calculated in three annual incidence cohorts of HIV-negative adults 15 years of age or older (including nonstable residents, persons who migrated into the community, and persons who migrated out) who had repeat annual HIV testing. At year 1, the analysis included 52,474 persons, representing 51,975 person-years of follow-up; at year 2, the analysis included 55,531 persons, representing 53,371 person-years of follow-up; at year 3, the analysis included 58,145 persons, representing 52,567 person-years of follow-up. For incident infections, the date of infection was imputed as the midpoint of the time between repeat HIV tests.
The relative rate (year 3 vs. year 1) was based on Poisson generalized estimating equations with an exchangeable covariance matrix, with adjustment for age, sex, and mobility (i.e., at least 1 month of the previous year spent outside the community).
COMMUNITY HEALTH OUTCOMES
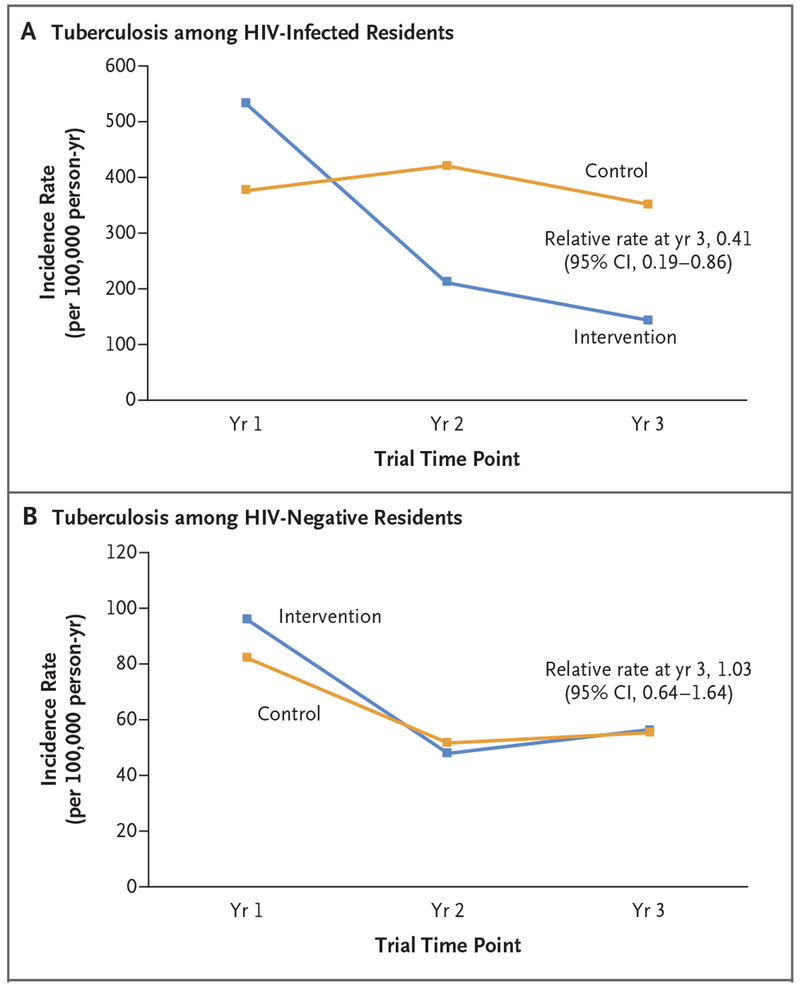
The cumulative probability of death due to illness by year 3 among adults who were HIV-positive at baseline (Fig. S17 in the Supplementary Appendix) was 23% lower in the intervention group than in the control group (3% vs. 4%; 0.99 deaths per 100 person-years vs. 1.29 deaths per 100 person-years; relative risk, 0.77; 95% CI, 0.64 to 0.93). The overall rate of death due to illness among all adults (irrespective of HIV infection) was 0.51 deaths per 100 person-years in the intervention group and 0.56 deaths per 100 person-years in the control group (relative rate, 0.90; 95% CI, 0.79 to 1.02). Among persons who were HIV-positive at baseline, the cumulative probability of incident tuberculosis or death due to illness by year 3 was 21% lower in the intervention group than in the control group (4% vs. 5%; 1.19 events per 100 person-years vs. 1.50 events per 100 person-years; relative risk, 0.79; 95% CI, 0.67 to 0.94); among persons with a CD4+ T-cell count of 500 or fewer per cubic millimeter, the cumulative probability was 29% lower in the intervention group than in the control group (5% vs. 7%; 1.62 events per 100 person-years vs. 2.31 events per 100 person-years; relative risk, 0.71; 95% CI, 0.56 to 0.88). Effects were similar when persons with an unknown HIV status at baseline were included in the analysis (relative risk, 0.80; 95% CI, 0.69 to 0.91) (Fig. S18 in the Supplementary Appendix). In a post hoc comparison of the two trial groups, the incidence rate of tuberculosis at 3 years among persons who were HIV-positive at baseline was 59% lower in the intervention group than in the control group (relative rate, 0.41; 95% CI, 0.19 to 0.86) (Fig. 3A), but the incidence rate of tuberculosis at 3 years did not differ significantly between the groups among persons who were HIV-negative at baseline (Fig. 3B).
Figure 3. Incidence of Tuberculosis over Time.
Panel A shows the incidence rates of tuberculosis over time among residents who were HIV-positive at baseline and who had not received a diagnosis of active tuberculosis at baseline (13,430 residents). Panel B shows the incidence rates of tuberculosis over time among residents who were HIV-negative at baseline and who had not received a diagnosis of active tuberculosis at baseline (121,604 residents). For both analyses, data for person-time at risk were censored at the time of migration out of the community, death, or diagnosis of active tuberculosis, and the trial groups were compared at 3 years with the use of community-level targeted maximum likelihood estimation.
At 3 years, among adults with prevalent hypertension, the percentage who achieved control of their hypertension was 26% higher in the intervention group than in the control group (47% vs. 37%; relative prevalence, 1.26; 95% CI, 1.15 to 1.39). Among persons who were HIV-positive and had hypertension at baseline, the percentage of persons who had both HIV viral suppression and control of hypertension was 22% higher in the intervention group than in the control group (72% vs. 59%; relative prevalence, 1.22; 95% CI, 1.08 to 1.37). Similar results were observed among persons who had diabetes or hypertension. (Details are provided in Figs. S19 through S21 in the Supplementary Appendix.)
DISCUSSION
The 3-year cumulative incidence of HIV infection was not significantly lower in the intervention communities than in the control communities in rural Uganda and Kenya after a population-level multidisease approach to HIV testing was implemented in all communities. However, the risk of death and the risk of tuberculosis or death among HIV-infected adults, as well as the prevalence of uncontrolled hypertension in all adults, were approximately 20% lower in the intervention communities than in the control communities.
In the assessment of a universal HIV test-and-treat strategy that used a community-based, multidisease approach, HIV testing was performed in 90% of adult residents in the intervention group at baseline, a percentage that increased to 94% by year 2; the time to initiation of ART was shorter in the intervention group than in the control group; and the percentage of HIV-infected persons with viral suppression in the intervention group exceeded both the percentage who had viral suppression in the control group and the UNAIDS target of 73%. The success of our testing approach was also reflected in the control group, in which baseline HIV testing coverage was 91%, which provided an opportunity for persons to engage in HIV care and access ART as eligibility expanded.
We did not detect a significant difference between the groups in the cumulative incidence of HIV infection despite the fact that the percentage of HIV-infected persons with viral suppression was 15% higher in the intervention group than in the control group after 3 years. The most likely explanation is that our active control with comprehensive baseline HIV testing and implementation of near-universal ART eligibility 1 year after the start of our trial increased the prevalence of population-level viral suppression from 42% to 68%, which consequently reduced the difference between the groups in the number of persons who were capable of transmitting HIV during the trial.4 Other possible explanations include infections from outside communities, outbreaks of acute HIV infection, and infection sources from a small subgroup of persons who had unsuppressed viral load — hypotheses that can be tested in future phylogenetic studies.13,14
Although no significant difference was observed between the groups in the 3-year cumulative incidence of HIV infection, the annual incidence of HIV infection in the intervention group declined by 32% between the first and third years. These data support accumulating evidence from other large universal test-and-treat and cohort studies in sub-Saharan Africa that population-level increases in viral suppression are associated with a reduction in the incidence of HIV infection.15–19 Because our trial included an active control group, we were unable to directly quantify the contribution of the SEARCH treatment intervention to the decline in HIV incidence. However, a mathematical model of SEARCH communities suggested that the intervention resulted in a lower incidence than that in a nonactive control; modeled HIV incidence in the third year was 0.4% with the SEARCH intervention, an incidence that was close to the observed incidence of 0.3% and that was 43% lower than the incidence of 0.7% that was predicted by the model without any SEARCH activities.20 We observed marked regional variations in the incidence of HIV infection from the first year to the third year, including a 46% decline in Kenya, where the prevalence of HIV infection was highest, and virtually no change in western Uganda, where the contribution of lower male circumcision coverage and migration of HIV-infected persons into the area is under investigation. In addition, declines in the incidence of HIV infection were less pronounced among women than among men, despite a high prevalence of viral suppression among men in a region in which HIV transmission to women occurs primarily through heterosexual encounters; both biologic and behavioral factors may have played a role.
We hypothesized that the SEARCH intervention would improve community health outcomes. Indeed, by year 3, we observed a cumulative probability of death among HIV-infected persons that was 23% lower in the intervention group than in the control group, an annual incidence rate of tuberculosis among HIV-infected persons that was 59% lower in the intervention group than in the control group, and a prevalence of control of hypertension in the overall population that was 26% higher in the intervention group than in the control group. We attribute these gains to our multidisease care model and patient-centered delivery of universal ART, which accelerated ART initiation among persons who had not been receiving treatment across all strata of CD4+ T-cell counts, including the stratum of less than 350 cells per cubic millimeter, the level at which the risk of death and tuberculosis are highest and at which all persons in both trial groups were eligible for immediate treatment.21 Our trial provides evidence that the way in which care is delivered can affect clinical outcomes, including death, tuberculosis, and hypertension, during efforts that are directed at HIV elimination.22–24
This trial had several limitations. First, because the control group had near-universal baseline HIV testing, we were unable to directly compare our intervention with the standard-of-care approaches used in the two countries. However, our data suggest that additional annual testing did not further reduce the incidence of HIV infection. Second, rapid implementation of expanding ART eligibility may have limited our ability to detect a significant difference between the trial groups, although universal treatment is now the global standard and, hence, the relevant comparison. Finally, results for the end point of tuberculosis relied on registry data, and results for the end point of death relied on information from family members or community members; however, ascertainment of these end points did not differ substantially between the groups.
An estimated 680 million persons will reside in rural sub-Saharan Africa by 2020. The SEARCH community health model is one approach to the reduction of HIV-associated deaths, tuberculosis, and other chronic diseases in rural sub-Saharan Africa, an approach that is in step with the United Nations Sustainable Development Goals. Our cost for a one-time health campaign was within the range of other mobile HIV testing approaches, and hypertension and diabetes testing added only $1 (U.S.) per person.25 Similarly, our cost for patient-centered care was similar to that reported by large funding agencies.26 The fact that the percentage of HIV-infected persons with viral suppression in our trial exceeded the UNAIDS target of 73% is an important step forward, but reducing the incidence of HIV infection to below 0.1% will require additional approaches to treatment and prevention that may be enhanced by a broader multidisease model incorporating cost and care delivery.27,28
Supplementary Material
Acknowledgments
Supported by the Division of AIDS, National Institute of Allergy and Infectious Diseases of the National Institutes of Health (awards U01AI099959, UM1AI068636, and R01 AI074345-06A1); the President’s Emergency Plan for AIDS Relief; and Gilead Sciences, which provided tenofovir–emtricitabine (Truvada) in kind.
Dr. Koss reports receiving grant support, paid to the University of California, San Francisco, from Gilead Research Scholars Program in HIV; and Dr. Rooney, being employed by and holding stock in Gilead Sciences. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the Ministry of Health of Uganda and the Ministry of Health of Kenya; our research teams and administrative teams in San Francisco, Uganda, and Kenya; collaborators and advisory boards; and especially all the communities and participants involved in the trial.
APPENDIX
The authors’ full names and academic degrees are as follows: Diane V. Havlir, M.D., Laura B. Balzer, Ph.D., Edwin D. Charlebois, Ph.D., M.P.H., Tamara D. Clark, M.P.H., Dalsone Kwarisiima, M.B., Ch.B., M.P.H., James Ayieko, M.B., Ch.B., M.P.H., Jane Kabami, M.P.H., Norton Sang, M.A., Teri Liegler, Ph.D., Gabriel Chamie, M.D., M.P.H., Carol S. Camlin, Ph.D., M.P.H., Vivek Jain, M.D., M.A.S., Kevin Kadede, M.A., Mucunguzi Atukunda, M.B., Ch.B., M.A.S., Theodore Ruel, M.D., Starley B. Shade, Ph.D., M.P.H., Emmanuel Ssemmondo, M.B., Ch.B., M.P.H., Dathan M. Byonanebye, M.B., Ch.B., M.Med., Florence Mwangwa, M.B., Ch.B., M.P.H., Asiphas Owaraganise, M.B., Ch.B., Winter Olilo, B.S., Douglas Black, B.A., Katherine Snyman, M.S., Rachel Burger, M.H.S., Monica Getahun, M.P.H., Jackson Achando, M.A., Benard Awuonda, B.Sc., Hellen Nakato, B.Sc., Joel Kironde, B.B.L.T., Samuel Okiror, B.B.L.T., Harsha Thirumurthy, Ph.D., Catherine Koss, M.D., Lillian Brown, M.D., Ph.D., Carina Marquez, M.D., M.P.H., Joshua Schwab, M.S., Geoff Lavoy, Albert Plenty, M.S., Erick Mugoma Wafula, B.Sc., Patrick Omanya, B.Sc., Yea-Hung Chen, Ph.D., James F. Rooney, M.D., Melanie Bacon, R.N., M.P.H., Mark van der Laan, Ph.D., Craig R. Cohen, M.D., M.P.H., Elizabeth Bukusi, M.B., Ch.B., M.Med., M.P.H., Ph.D., Moses R. Kamya, M.B., Ch.B., M.Med., M.P.H., Ph.D., and Maya Petersen, M.D., Ph.D.
The authors’ affiliations are as follows: the Division of HIV, Infectious Diseases, and Global Medicine, Department of Medicine (D.V.H., T.D.C., T.L., G.C., V.J., D.B., K.S., C.K., L.B., C.M.), the Division of Prevention Science, Department of Medicine (E.D.C., S.B.S., A.P.), the Department of Obstetrics, Gynecology, and Reproductive Sciences (C.S.C., R.B., M.G., C.R.C.), and the Division of Infectious Diseases, Department of Pediatrics (T.R.), University of California, San Francisco, and the San Francisco Department of Public Health (Y.-H.C.), San Francisco, the Division of Epidemiology and Biostatistics, the School of Public Health, University of California, Berkeley (J.S., M.L., M.P.), and Gilead Sciences, Foster City (J.F.R.) — all in California; the School of Public Health and Health Sciences, University of Massachusetts, Amherst (L.B.B.); the Infectious Diseases Research Collaboration (D.K., J. Kabami, M.A., E.S., D.M.B., F.M., A.O., H.N., J. Kironde, S.O., G.L.) and the School of Medicine, Makerere University (M.R.K.), Kampala, Uganda; Kenya Medical Research Institute, Nairobi (J. Ayieko, N.S., K.K., W.O., J. Achando, B.A., E.M.W., P.O., E.B.); Perelman School of Medicine, University of Pennsylvania, Philadelphia (H.T.); and the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD (M.B.).
Footnotes
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the President’s Emergency Plan for AIDS Relief, or Gilead Sciences.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
REFERENCES
- 1.The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015;373:808–22. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global AIDS update 2018: miles to go. Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS), 2018. [Google Scholar]
- 5.Fox MP, Rosen S. Retention of adult patients on antiretroviral therapy in low- and middle-income countries: systematic review and meta-analysis 2008-2013. J Acquir Immune Defic Syndr 2015;69:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy M, Czaicki N, Holmes C, et al. Understanding sustained retention in HIV/AIDS care and treatment: a synthetic review. Curr HIV/AIDS Rep 2016;13:177–85. [DOI] [PubMed] [Google Scholar]
- 7.Chingono A, Lane T, Chitumba A, Kulich M, Morin S. Balancing science and community concerns in resource-limited settings: Project Accept in rural Zimbabwe. Clin Trials 2008;5:273–6. [DOI] [PubMed] [Google Scholar]
- 8.Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: what’s new. Geneva: World Health Organization, November 2015. (http://www.who.int/hiv/pub/arv/15249_HIVTreatementandCare_PolicybriefforWEB.pdf). [Google Scholar]
- 9.Chamie G, Clark TD, Kabami J, et al. A hybrid mobile approach for populationwide HIV testing in rural east Africa: an observational study. Lancet HIV 2016;3(3): e111–e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain V, Liegler T, Kabami J, et al. Assessment of population-based HIV RNA levels in a rural east African setting using a fingerprick-based blood collection method. Clin Infect Dis 2013;56:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwarisiima D, Kamya MR, Owaraganise A, et al. High rates of viral suppression in adults and children with high CD4+ counts using a streamlined ART delivery model in the SEARCH trial in rural Uganda and Kenya. J Int AIDS Soc 2017; 20:Suppl 4:21673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balzer LB, van der Laan MJ, Petersen ML. Adaptive pre-specification in randomized trials with and without pair-matching. Stat Med 2016;35:4528–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller WC, Rosenberg NE, Rutstein SE, Powers KA. Role of acute and early HIV infection in the sexual transmission of HIV. Curr Opin HIV AIDS 2010;5:277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinkerton SD. How many sexually-acquired HIV infections in the USA are due to acute-phase HIV transmission? AIDS 2007;21:1625–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grabowski MK, Serwadda DM, Gray RH, et al. HIV prevention efforts and incidence of HIV in Uganda. N Engl J Med 2017;377:2154–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borgdorff MW, Kwaro D, Obor D, et al. HIV incidence in western Kenya during scale-up of antiretroviral therapy and voluntary medical male circumcision: a population-based cohort analysis. Lancet HIV 2018;5(5):e241–e249. [DOI] [PubMed] [Google Scholar]
- 17.PHIA Project. Eswatini. New York: ICAP at Columbia University, 2018. (https://phia.icap.columbia.edu/countries/swaziland/). [Google Scholar]
- 18.Hayes RJ, Donnell D, Floyd S, et al. Effect of universal testing and treatment on HIV incidence — HPTN 071 (PopART). N Engl J Med 2019;381:207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makhema J, Wirth KE, Pretorius Holme M, et al. Universal testing, expanded treatment, and incidence of HIV infection in Botswana. N Engl J Med 2019;381:230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jewell B, Balzer L, Clark TD, et al. Modeling projected HIV incidence in the SEARCH study of treatment as prevention in East Africa. Presented at the 22nd International AIDS Conference (AIDS), Amsterdam, July 23–27, 2018:TUPEC297. abstract. [Google Scholar]
- 21.Lawn SD, Little F, Bekker LG, et al. Changing mortality risk associated with CD4 cell response to antiretroviral therapy in South Africa. AIDS 2009;23:335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Global Health Observatory (GHO) data: tuberculosis (TB). Geneva: World Health Organization, 2018. (http://www.who.int/gho/tb/en/). [Google Scholar]
- 23.Nugent R, Bertram MY, Jan S, et al. Investing in non-communicable disease prevention and management to advance the Sustainable Development Goals. Lancet 2018;391:2029–35. [DOI] [PubMed] [Google Scholar]
- 24.Gómez-Olivé FX, Ali SA, Made F, et al. Regional and sex differences in the prevalence and awareness of hypertension: an H3Africa AWI-Gen study across 6 sites in sub-Saharan Africa. Glob Heart 2017;12: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang W, Chamie G, Mwai D, et al. Implementation and operational research: cost and efficiency of a hybrid mobile multidisease testing approach with high HIV testing coverage in East Africa. J Acquir Immune Defic Syndr 2016;73(3):e39–e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shade SB, Osmand T, Luo A, et al. Costs of streamlined HIV care delivery in rural Ugandan and Kenyan clinics in the SEARCH Study. AIDS 2018;32:2179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horton R Offline: can the Global Fund survive? Lancet 2018;392:14. [DOI] [PubMed] [Google Scholar]
- 28.Bekker LG, Alleyne G, Baral S, et al. Advancing global health and strengthening the HIV response in the era of the Sustainable Development Goals: the International AIDS Society-Lancet Commission. Lancet 2018;392:312–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.