Figure 3.
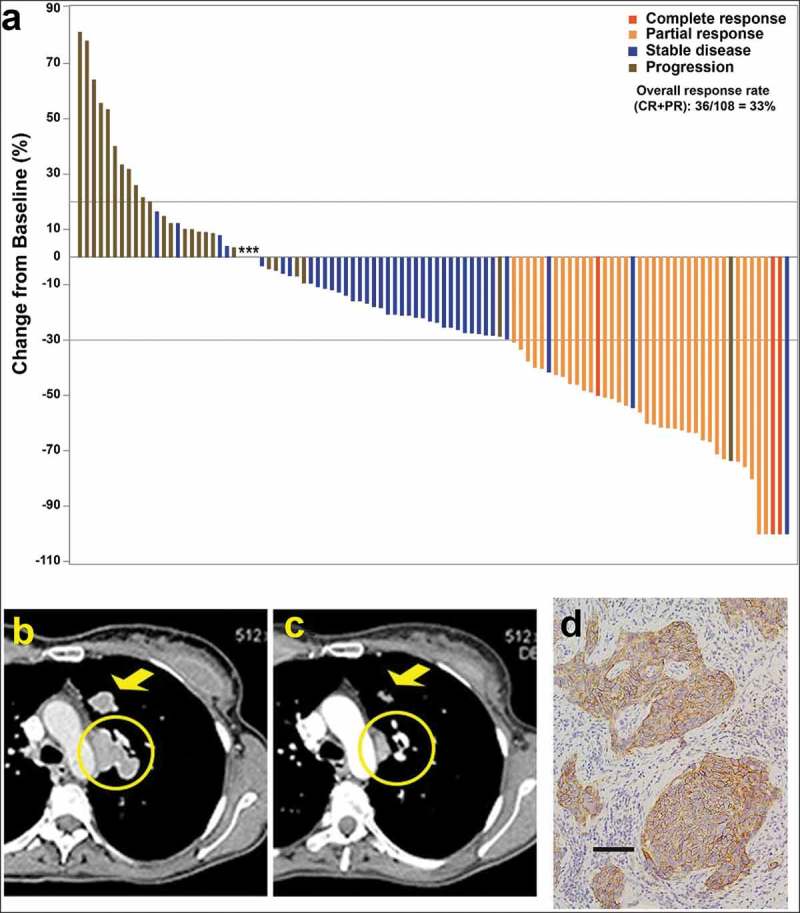
(a) Waterfall plot illustrating the best response data in 108 triple-negative breast cancer patients treated with sacituzumab govitecan (adapted from Bardia et al.,50 with permission). (b–d) Tumor shrinkage by CT in triple-negative breast cancer patient given sacituzumab govitecan (from Bardia et al.38 with permission). Case study: A 48-year-old woman with an initial diagnosis of triple-negative breast cancer in received four prior lines of treatment (including an anti-PD-L1 immune checkpoint inhibitor) and presented with lung and lymph node metastases at enrollment. She achieved a partial response that started 1.7 months after initiation of treatment with sacituzumab govitecan, with the best response of 54% reduction at 9.0 months and progression occurring at 14.4 months (partial response duration, 12.7 months). (b) Baseline image of two of the three target lesions: a 24 x 19-mm left-upper-lung mass (arrow) and a mediastinal lymph node (17 x 29-mm; circle). (c) After 16 doses, these two target lesions decreased to 13 × 7 and 9 × 19 mm, respectively. (d) TROP-2 expression in an archived tumor specimen by immunohistochemistry that shows 1+ to 2+ staining (overall, 2+).
