Abstract
Atrial fibrillation (AF) is the commonest arrhythmia, yet the mechanisms of its onset and persistence are incompletely known. Although techniques for quantitative assessment have been investigated, there have been few attempts to integrate this information to advance disease treatment protocols. In this review, key quantitative methods for AF analysis are described, and suggestions are provided for the coordination of the available information, and to develop foci and directions for future research efforts. Quantitative biologists may have an interest in this topic in order to develop machine learning and tools for arrhythmia characterization, but they may perhaps have a minimal background in the clinical methodology and in the types of observed events and mechanistic hypotheses that have thus far been developed. We attempt to address these issues via exploration of the published literature. Although no new data is presented in this review, examples are shown of current lines of investigation, and in particular, how electrogram analysis and whole-chamber quantitative modeling of the left atrium may be useful to characterize fibrillatory patterns of activity, so as to propose avenues for more efficacious acquisition and interpretation of AF data.
Keywords: atrial fibrillation, automaton, dominant frequency, electrograms, model
Overview
Atrial fibrillation (AF) is a common heart arrhythmia, yet how it is initiated and maintained is mostly unknown [1]. The risk of AF recurrence and thromboembolism may be mitigated with antiarrhythmic drugs and anticoagulant agents, respectively. However, the success rate with pharmacologic treatment is relatively low, and recurrences are common [2]. Catheter ablation of suspected atrial triggers in arrhythmogenic regions such as the pulmonary veins can be done in the electrophysiology laboratory. Yet, any arrhythmogenic regions residing outside of the pulmonary veins may be difficult to detect and localize directly, since such triggers are not always evident during electrophysiologic testing. Quantitation of electrograms acquired from the heart surface, and modeling of the myocardial substrate, have been proposed and have shown some promise to visualize suspected drivers, and to describe AF characteristics under certain conditions. To the present time however, the success of such techniques is in rather simple problem-solving, such as to determine the significant differences in the cardiac parameters of patients with paroxysmal versus persistent type of arrhythmia [3]. In ongoing work, major goals include the determination as to whether quantitation can be utilized to estimate and predict the progression of AF and its causal mechanisms, as well as to suggest a more efficacious ablation strategy, perhaps focused on the individual patient, as has been the subject of precision medicine efforts [4]. In this review, issues pertaining to the quantitative interpretation of atrial fibrillation data are described and discussed, and suggestions are made for improvement, such that testable hypotheses and techniques for understanding the mechanisms of AF and best ablation strategies can be developed.
Background
To enhance techniques for obtaining salient information concerning the mechanisms of AF initiation and perpetuation, and to best locate catheter ablation sites at arrhythmogenic zones for treatment, there is a need for improving the methods of data acquisition and interpretation of the acquired data, which includes heart surface recordings (i.e., electrogram signals). To this end, an increase in the spatiotemporal resolution of local heart surface recordings, better analysis of these electrograms, and development of quantitative models more representative of AF phenomena are important. Currently, AF drivers residing outside of the pulmonary vein antrums are difficult to detect in the electrophysiology laboratory [5], and many observed AF events are not well reproduced by quantitative modeling [6]. Furthermore, a major difficulty in targeting sites for ablation is that the sources of arrhythmia at onset are mostly unknown in terms of their dynamic properties and location [7]. Early whole-chamber as well as molecular-level quantitative models have been constructed to simulate the electrophysiologic conditions occurring during AF [8–15]. By tweaking model parameters, it might be possible to develop hypotheses regarding the connection between model-simulated events and corresponding actual AF events. Yet, these models may have limited accuracy in reproducing AF episodes, in part because the important electrophysiologic parameters to incorporate, and the correct values for parameterization, are not entirely known [16]. If it were possible to localize AF drivers from quantitative analysis, such as from heart surface electrogram time series features and spectral analysis [17], to develop higher resolution acquisition and mapping techniques [18], and to better interpret these maps for understanding AF mechanisms, then the timeliness and efficacy of clinical treatment procedures could potentially be improved.
One difficulty in improving quantitative analysis is that the etiology of AF is partially unknown [19]. There are however, two factors likely to be important in its onset and perpetuation [7]. The basic pathophysiological mechanisms of AF are known to be focal ectopic activity and reentry [10]. Electrical impulses typically originating in the pulmonary veins or in the pulmonary vein antrums, as well as in the left atrial free wall, can overwhelm the normal sinus rhythm activation sequence. These sources may act as premature electrical stimuli so that unexpected, or “rogue”, electrical activation wavefronts, commence in the left atrial myocardium. These stimuli can therefore be considered drivers of AF, but they are focal, in that the electrical impulses originate from point sources, outside the normal sinus rhythm process, to generate irregular and often random electrical activation wavefronts. The coupling interval between any such pulses can sometimes or even frequently be less than the refractory period in certain atrial myocardial regions, resulting in functional conduction block [20]. Even if the periodicity of any rogue wave emanation is longer than the tissue refractory period, if the timing precedes the recovery of excitability after normal sinus rhythm activation, functional block would also occur.
Areas of functional block caused by focal stimuli can lead to rotational electrical activity, as a rogue wave bifurcates around a refractory region, and then may propagate through it in a circular pattern after recovery of excitability [21]. Rotational features so formed also generate rogue waves, and are also likely to be important in the onset and maintenance of AF. Localized focal sources and rotors may be found in patients with any type of AF [22]. Panoramic contact mapping has also provided evidence that sustained AF may be largely due to focal sources and stable electrical rotors in either atrium [23]. Ablation at these sites can terminate or substantially organize the arrhythmia prior to any additional ablation.
The electrophysiologic conditions conducive to the formation of rotational patterns of electrical activity include the presence of areas of slow conduction velocity and/or relatively short refractory period along the prospective reentrant circuit pathway [24]. These factors result in a short wavelength, which is defined as the product of the local refractory period multiplied by the local conduction velocity, i.e.:
At short wavelengths, a finite excitable gap, the time interval during which the local tissue has recovered excitability prior to the next activation cycle, is more likely to be maintained [25]. Thus, the activation wave can be perpetuated in a circuitous path without encountering refractoriness to electrical conduction that would otherwise block its continued propagation.
In patients with ongoing AF, the remodeling of myocardial tissue can occur on both a gross and microscopic scale [26, 27]. Dispersion of action potential duration (APD) is a manifestation of remodeling that is likely important for onset of reentry in AF [28]. Furthermore, microscopic structural changes to electrophysiologic alterations in ion channel function, gap junctional connection, and variability in other cellular structures and parameters may lead to fibrosis [29] and metabolic derangement [30]. An important pathologic alteration observed in persistent AF patients is tissue fibrosis [27]. Fibrosis is a source of anatomical conduction block [31], which can slow the activation wave and cause circuitous pathways of activation, thereby enabling a contribution to the persistence, complexity, and progression of the fibrillatory process.
The two main observed patterns of atrial rotational activity are the presence of vortices, which swirl about a small unexcitable core [22,23,32], and can consist of one, two, or even several reentrant circuit loops in tandem, and freestanding wavelets [33, 34], which are activation wavefronts with distinct ends that travel about larger impediments to conduction, such as regions of fibrosis or an ablation lesion. For both of these rotational type patterns, the leading edge of the activation wave reenters previously excited areas after their recovery of excitability, thus following a circuitous pattern. Tissue remodeling often occurs when rotational features with rapid activation are present in the atrial substrate to drive AF. The remodeling process may include an increased release of calcium ions from the sarcoplasmic reticulum [35], which reduces the APD, and correspondingly shortens the refractory period for electrical conduction. AF is associated with a decrease in adaptation of APD to rate as well as to changes in atrial conduction velocity [36]. These phenomena can contribute to further perpetuation of AF (i.e., ‘AF begets AF’ [37]) since the wavelength λ is further diminished.
To terminate AF and prevent its recurrence, sources of premature stimuli should be ablated or electrically isolated [38]. However, whether or not anatomical locations which serve as sites of formation for rotational features should also be ablated or electrically isolated, is difficult to determine since they are not readily detectable [39]. Furthermore, AF drivers can be intramurally positioned [40], which would make localization problematic for achieving a successful ablation. AF recurrence could be prevented by removing all ectopic foci and rotational features, both of which are drivers of AF. Yet, targeting triggers outside of pulmonary vein isolation is not currently the standard of care [41]. Since the goal of successful AF treatment in the electrophysiology laboratory is the removal of all drivers, guidance for successful targeting has not yet reached a consensus and requires further study.
The complexity of AF and its mechanism of onset by premature stimulation, and perpetuation by focal and rotational activity, stem from the presence of multiple sites where drivers may appear either sequentially or simultaneously in time. Although multiple reentrant circuits can be present, they may not be fixed in location over long time periods. The presence of any rotational pathways are typically difficult to localize in AF, owing to the lack of long-term repeatability of the temporal activation pattern [42] so that presently, radiofrequency ablation of these regions has unknown efficacy. AF is occasionally a progressive condition [43], so that arrhythmia duration without interruption tends to increase over time.
Acquiring clinical AF data for quantitation
When patients with paroxysmal AF are subject to electrophysiologic study, the pulmonary veins are first electrically isolated, and the ability of the ablation lesions to block propagation is assessed. To eliminate drivers in the atrial free wall region, it would then be necessary to reinduce AF by programmed electrical stimulation, followed by localization of any such suspected drivers. Yet, a particular stimulus location and pulse train may result in AF induction on one instance, but fail to induce AF for a subsequent instance. Furthermore, stimuli applied to one location may result in AF onset, yet from another location the same stimulus train may fail to induce AF. These points are illustrated in Figure 1. The process of AF induction in the electrophysiology laboratory is tedious and time consuming, because there is no set stimulus pattern which results in AF onset. Thus the induction characteristics for AF are not highly repeatable, and furthermore, differences in the characteristics of the activation pattern during AF may occur on different inductions, making more difficult the drawing of conclusions from quantitative efforts. Moreover, during clinical study, paroxysmal AF can terminate while ablation is ongoing. However, the catheter tip location and the magnitude of energy delivery at termination may be unrelated to the AF process, with further induction and ablation being necessary to prevent arrhythmia recurrence (Figure 2, left side). Thus the characteristics of the acquired heart surface electrogram signal at the termination site is likely to be mostly or entirely irrelevant to the detection of paroxysmal AF drivers, another problem posed for quantitative analysis. After AF termination, programmed electrical stimulation at many sites, and pulsed extrastimuli patterns, may be utilized to establish whether AF can be reinduced, and to test whether or not other atrial arrhythmias such as atrial flutter or tachycardia are inducible [44].
1.
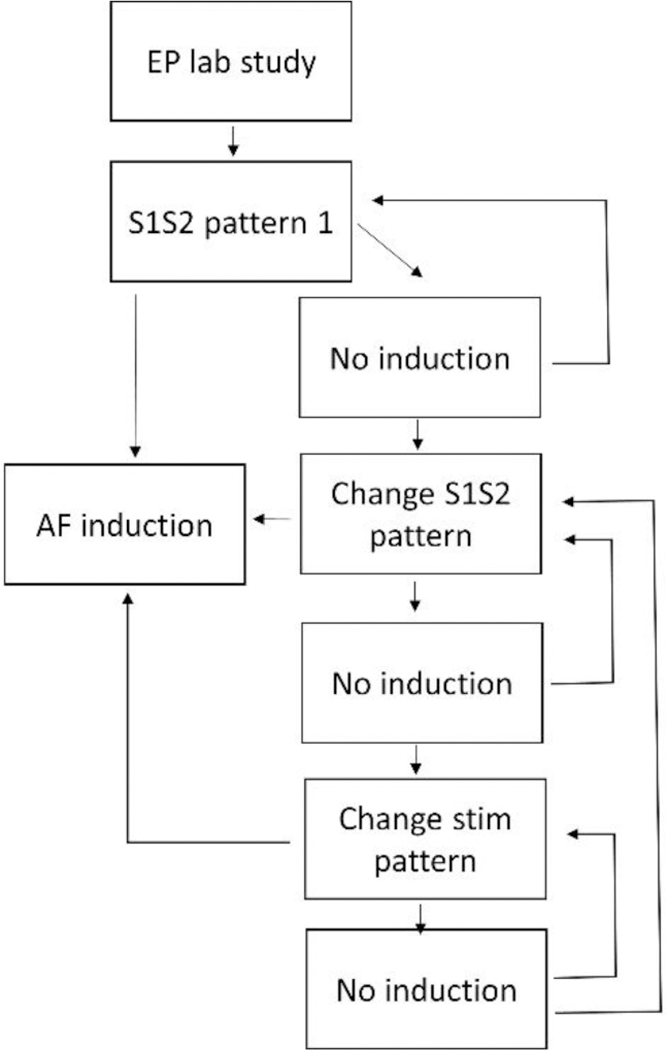
Block diagram of the procedure to induce atrial fibrillation in paroxysmal patients. Various coupling intervals of S1 and S2 pulses are utilized until AF is induced from a particular programmed stimulation site. If these attempts fail, IV isuprel may be administered, and the stimulating electrode is moved to another location and the process repeated. The procedure is repetitive and time-consuming, because little is known regarding the best stimulation sequence to employ, and the events occurring during stimulation are not very repeatable.
2.
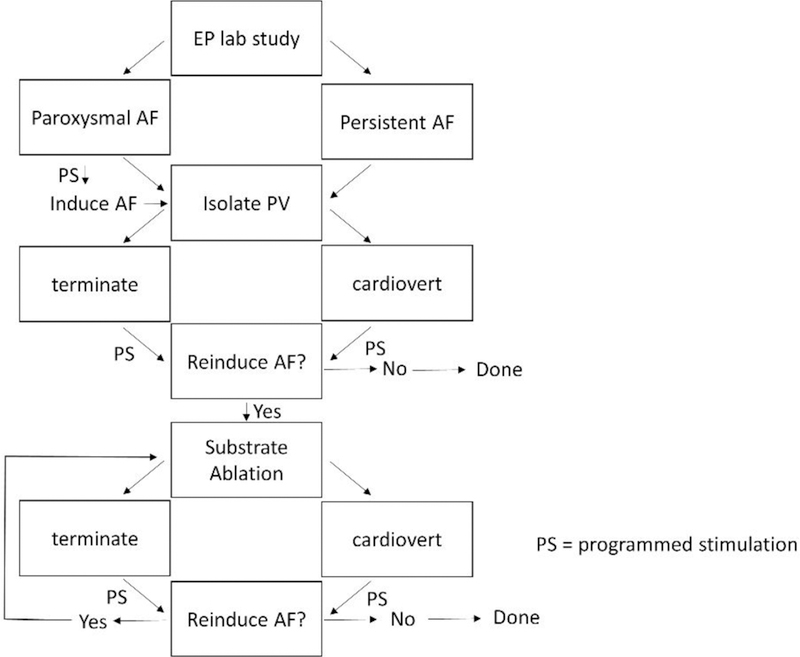
Block diagram showing how drivers are eliminated in paroxysmal and persistent AF. Even after ablating suspected driver locations, atrial fibrillation may still need to be terminated in both types of patients. Once atrial fibrillation is terminated, further induction of arrhythmia is attempted, following the paradigm shown in Figure 1. The process is repeated until atrial fibrillation can no longer be induced. The procedure is time-consuming and not always successful, because what to ablate and where to ablate to eliminate drivers is often uncertain.
In contrast, for patients with persistent AF, spontaneous termination of arrhythmia during ablation is rare (Figure 2, right side). When termination does occur, the ablation site is likely to be important to arrhythmia maintenance; therefore the time and frequency characteristics of electrograms recorded in the area can potentially be useful markers of persistent AF drivers. After ablation, and after cardioversion when it is needed for termination, the process may be repeated until AF is no longer inducible, and if successful, it can be used as a procedural endpoint. However, in both paroxysmal and persistent patients, AF may recur after many weeks or months due to the reestablishment of conduction pathways across ablation lesions [45], and due to the development of additional microstructural areas where drivers can form. Hence, detecting driver sites through quantitation may involve finding both current locations that are capable of driving arrhythmia, as well as finding locations that are evolving to become drivers. Detection of these latter locations would only be possible if substrate features were to be identified in the data that are predictive of future events, an ongoing research effort.
When surface AF electrograms are acquired with a standard catheter having a single electrode at its tip, there is often a preference for using a bipolar configuration for quantitation, because the far-field signal, motion artifact, and electrical noise are then mostly eliminated by subtraction. Yet, bipolar recordings are difference signals, and their amplitude and shape depend upon activation wavefront direction, so that they must be used with some caution [46].
Furthermore, interelectrode distance also influences bipolar voltage [47]. Noncontact mapping is feasible to estimate heart surface electrogram characteristics with high spatial resolution, and the virtual electrograms obtained can even be used to estimate local frequency characteristics [48]. Moreover, contact catheters having ten bipolar electrodes are becoming more frequently used for AF data acquisition, and even basket catheters with many recording electrodes, as well as higher density mapping catheters, are now utilized in the electrophysiology lab. A difficulty with these multichannel devices is that they can be bulky, and some electrodes may not reside in contact with the endocardial surface [49]. The signals so obtained will then be an average of the activity in the whole atrial chamber, which will not be reflective of nearby heart surface activation times. Recently introduced multichannel recording devices are beginning to offer greater flexibility, greater spatial resolution, and minimized size and weight, for ease of use and improved surface contact [50], which could prove assistive to improve acquisition quality for future studies. Body surface signals can localize left and right atrial high-frequency rotors in AF [51]. Body surface mapping can also be used to identify reentrant and focal sources important to driving and perpetuating AF [52]. The spectral analysis of such AF body surface recordings enables a noninvasive characterization of the global distribution of the atrial frequency and identification of areas with highest frequency, enabling the possibility of personalized diagnosis and treatment [53].
Dimensionality of the acquired atrial data
Although AF signals are typically acquired in clinical studies from the endocardial heart surface only, which can be considered as a two-dimensional surface, a third dimension, the Z or thickness axis, has significance for understanding disorganized electrical activity [11, 54]. Rotational sources are likely to be in part three-dimensional. Such pathways may have only a small directional component along the Z-axis, particularly at thinner portions of the atrium. Yet thicker wall regions exist in both normal and enlarged atria, thus enabling the establishment of a longer Z-axis pathway through which any reentrant path can proceed. Transmural reentrant features extend from epicardium to endocardium, and breakthrough of electrical impulses traveling along the Z-axis, originating from the epicardium, may occur, which can contribute to activation pattern complexity during AF [55]. Certain electrophysiologic parameters may vary according to wall thickness, and this relationship should be considered in future modeling efforts [56].
The atrial anatomy and thickness may affect electrical wave dynamics in AF [57]. The location of breakthrough sites and lines of functional block during incomplete reentry may be related to preferential propagation according to the underlying subendocardial muscle structure [58]. Based on the contribution of wall thickness, AF substrates are dynamic three-dimensional structures with a range of discordance between epicardial and endocardial tissues [59]. The complex three-dimensional atrial structure can play a major role in activation sequence during AF [58]. Where the atrial wall is relatively thick, small intramural reentrant circuits might possibly contribute to driving AF, and could be responsible for some of the observed heart surface phenomena [40, 60]. Reentry with an intramural axis of rotation (filament) will appear on the endocardial/epicardial surface as breakthrough. A reentrant circuit contained within the atrial wall whose filament stretches between opposite surfaces is transmural in scope. Heterogeneous atrial wall thickness and atrial stretch, in combination with ionic and anatomic remodeling caused by AF, are important to enable atrial scroll waves and maintenance of arrhythmia [61]. In the presence of stretch, meandering three-dimensional scroll waves anchor at regions of large spatial gradient in wall thickness. Greater intramural discontinuity in the form of higher fiber density, or presence of other irregularities in the microstructure, can also serve as formation and anchoring points for any such mid-myocardial circuits [40].
Electrogram quantitative analysis
To detect AF drivers, the amplitude, shape, and repetitiveness of electrogram signals acquired from the endocardial surface of the heart have been proposed as metrics. Typically, electrogram sequences of approximately eight seconds duration are utilized [62]. For spectral estimation, the Fourier transform (FT), an autoregressive model [63], or another estimator is commonly implemented [64]. The statistical stability of the electrogram signal over time is important for frequency analysis [65,66]. Several electrogram frequency parameters have been found useful to distinguish paroxysmal versus persistent AF type. The dominant frequency or DF, which is the largest nonharmonic spectral peak in the range 3–12 Hz, tends to be higher in persistent as compared to paroxysmal AF patients [67] with average values of 6.22Hz for persistent versus 5.67Hz for paroxysmal AF patients [68]. This reflects a tendency for shortened refractory period, due to remodeling, and therefore faster reactivation, in persistent AF. There is also a greater temporal variation in the DF in paroxysmal (1.00Hz) versus persistent AF patients (0.78Hz) [68]. Earliest AF activation is often located close to the highest DF site [69]. Another useful frequency parameter has been shown to be the dominant amplitude (DA), defined as the magnitude of the DF peak, which is measured on normalized signals. The DA tends to be of larger average magnitude in persistent (1.34 normalized millivolts) versus paroxysmal AF patients (1.09 normalized millivolts) [68]. Moreover, the dominant morphology (DM), stated as the shape of sequential electrogram segments with a segment length corresponding to the DF, has a mean temporal correlation of 0.62 for persistent versus 0.50 for paroxysmal AF electrograms [70]. Thus the repetitiveness of electrogram patterns increases and becomes more uniform in persistent as compared with paroxysmal AF [71]. These findings suggest that persistent AF recordings tend to exhibit a more temporally stable and repetitive activation pattern.
To further advance the quantitative methods for interpretation of clinical data, cardiac magnetic resonance has recently been utilized to localize atrial fibrosis, and thereby to determine the relationship between electrogram time series deflections and fibrosis caused by remodeling [72]. Any such relationship might be useful to expound upon the observation that fibrosis is often minimal or nonexistent in paroxysmal AF patients, yet common in persistent AF, from which it may lead to electrogram fractionation [73]. Electrogram fractionation, the appearance of multiple deflections and low amplitude features in the AF electrogram over long intervals, is common at some atrial regions. The electrogram shape may also be related to microarchitectural components [74, 75], though this is yet to be confirmed.
During induced AF, systolic interval shortening following either drift or acceleration of a rotational source has resulted in intermittent fibrillatory conduction and formation of fractionated electrograms at the posterior left atrial wall [69]. Shortening of AF cycle length tends to precede the development of electrogram fractionation [76]. Longer electrogram durations, often observed in fractionated signals, have been recorded more frequently in the left as compared with the right atrium. Activation maps during transitions to fractionation reveal areas of slowed conduction and unidirectional block [69]. Targeting fractionated electrograms has been found to not simply be atrial debulking. Ablating certain fractionated electrograms increases AF cycle length, suggesting that these areas are important in maintaining AF [77].
The extracellular electrograms recorded during AF can be difficult to interpret due to cycle-to-cycle variability in their amplitude and time duration [78]. To ameliorate, phase mapping has been introduced as a quantitative technique which represents signals in terms of their relative position within a cycle. It has been widely applied to the action potential as well as to unipolar electrogram data [78]. Phase analysis of AF has gained interest due to its ability to localize organized stable rotational drivers to target for therapy [79]. The phase maps highlight areas of high-curvature wavefronts and rotors. Algorithms have been developed for calculating phase from both unipolar and bipolar electrograms recorded during AF [78]. However for some activation patterns, phase mapping generates non-rotational singularity points and false rotors. The number of detected rotors depends in part upon the parameters of the phase detection algorithm and the number of electrodes used for mapping [80]. A double ring comprised of a 2 × 2 and 4 × 4 grid of electrodes to acquire phase mapping data has been found useful for robust rotor detection.
Electrogram quantitation implemented thus far has been shown to correspond to some measured clinical parameters. For example, the average electrogram frequency spectrum level is correlated with the duration of uninterrupted persistent AF prior to electrophysiologic study, and to left atrial volume, for both persistent and paroxysmal arrhythmia [81]. Moreover, the DA parameter tends to increase with increasing left atrial volume. Based on a regression analysis, it would therefore be possible to estimate AF duration and atrial volume a priori. Furthermore, in early-stage clinical studies, quantitative AF models have been successfully used to identify patients in which pulmonary vein isolation alone is not adequate for treatment of AF, and to suggest novel targets for ablation [6]. Hence, further development of electrogram quantitative methods should be promising to detect and estimate AF events and clinical parameters.
Molecular-level AF simulation
Molecular-level quantitative models can be important for the accurate simulation of AF. Any such models should include a consideration of all observed electrophysiologic phenomena. The elaborate molecular changes in AF are known to be directed primarily at protecting the myocyte from cellular stress [82]. However, this early protection occurs at the expense of electrophysiological changes that promote the long-term maintenance of AF. Occurrence of arrhythmia leads to alterations in potassium and calcium currents that likely cause a decreased APD, and a decreased APD rate adaptation [36]. Increased diastolic sarcoplasmic reticulum Ca2+ leak, and the related delayed after-depolarizations/triggered activity, promote cellular arrhythmogenesis in paroxysmal AF patients [83]. Sustained atrial activation and AF-induced electrical remodeling is associated with reductions in transient outward current (Ito), ultrarapid delayed rectifier current (IKur), and L-type calcium current (ICa,L) [8,84,85]. The reduction in ICa,L alone can account for many morphological features of AF action potentials. Chronic rapid activation of the substrate also alters atrial ion channel gene expression, changing ionic currents so as to promote AF onset. To study AF pathophysiology in order to determine the molecular basis and the contribution of ion currents, animal models have also been used [86].
Whole-chamber AF modeling
Although mathematical models of cardiac electrical excitation at the molecular level are accurate to reproduced observed electrophysiologic phenomena, they have become increasingly complex and computationally expensive [16]. It is difficult to simulate AF for long time periods using state-of-the-art molecular-level models at current computational speeds. Although cellular automata are limited in their ability to reproduce electrophysiologic parameters during AF, they may be useful to show general concepts. In the more sophisticated such models, a sensitivity to the previous rate of activation is imparted to form the restitution parameter of a cell [16]. In one such realistic model, the stochastic initiation of AF arising from bursts of spontaneous activation near the simulated pulmonary veins, and its spontaneous termination with dependence on past AF trajectory has been shown [16]. The design of an automata model of activation wavefront propagation on an anisotropic structure has been developed to mimic the branching network of heart muscle cells [11]. This integration has been utilized to demonstrate how AF emerges spontaneously when the transverse cell-to-cell coupling decreases [11]. It has also been used to elucidate the stochastic nature of progressive transversal uncoupling of muscle strands (e.g., due to fibrosis or gap junctional remodeling), as occurs with age, resulting in variability in AF episode onset time, frequency, duration, burden, and progression between individuals [12]. Using a simple cellular automata model of AF, a method by which re-entrant drivers can be located quickly and accurately using a collection of indirect electrogram measurements has been demonstrated [54]. These simplified representations of atrial electrical activity reduced computational cost.
Although further simplification of whole-chamber atrial electrophysiology is imperfect, it may be useful to show the timing of AF events [13–15]. As an example, after simulated premature stimulation on a 576 × 576 grid with anisotropy, a refractory gradient, and simulated nonconducting collagen fibers, it has been shown that synthetic fibrillatory activity can commence (Figure 3A). Early-to-late activation is colored from red to violet in the panel. Both rotational features and freestanding wavelets are evident. These features are in part formed and stabilize by the presence of simulated nonconducting fibers. Imparting simulated patch ablation lesions (black squares, Figure 3B), the remaining wavefronts after simulated ablation tend to reside further apart. If simulated linear ablation lesions are imparted to the grid (black lines, Figure 3C), wavefront spatial density also diminishes. The cause of this phenomenon, determined from timing considerations, is diagramized in Figure 4. A gradient of long-to-short refractory period exists from top-to-bottom. When a patch ablation lesion eliminates a rotational feature (Figure 4A), proximate rotational features then drive that region (Figure 4B–C), yet at a slower rate. A diminished activation rate occurs because the top driver spins slowly due to its longer refractory period, while the bottom driver spins more rapidly due to its short refractory period, but this latter event causes 2:1 conduction alternans into the center region where ablation was imparted. Similarly, when linear ablation lesions are applied to the center region, they shield portions of it from activation by its local driver, so that peripheral rotational features activate the center region instead, again at a slower rate. Similarly, during actual EP lab ablation, a number of studies have suggested that activation rate in the atrium decreases during catheter ablation [87,88].
3.
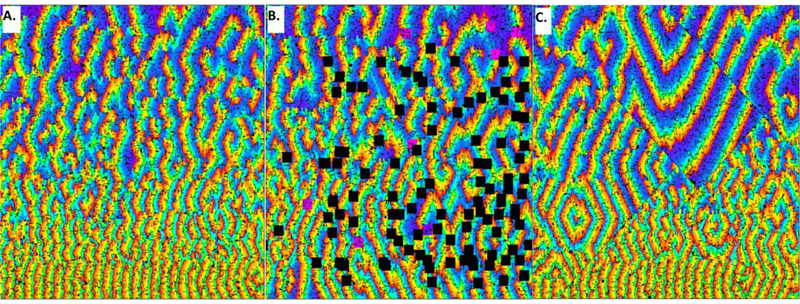
In this simulation, an automata grid is used to simulate fibrillatory activity. A refractory gradient of long to short (175 ms to 75 ms) is present from top to bottom, Panel A. There is 2:1 anisotropic conduction in the vertical versus horizontal directions. Minute fibers are evident as short thin black lines, randomly distributed about the grid to simulate fibrosis. The fibrillatory activity evident in panel A occurs prior to any simulated ablation. In panel B, patch ablation lesions of dimension 20×20 nodes were imparted, illustrated as black squares, at locations where vortices had been evident. There is a loss in the density of activation wavefronts in panel B, and a greater surface area of obstacles to conduction as compared with panel A. This causes prolongation of the mean global cycle length for grid activation, evident as a change in background color, with more blue and violet in panel B. Linear ablation (thin black lines marked by interruption in the activation wave direction and spacing) also impede or eliminate the propagation of local driver wavefronts (panel C). Prolonged activation intervals are evident due to the presence of the linear lesions, which can shield areas from their local drivers, as is particularly evident in the upper portion of panel C.
4.
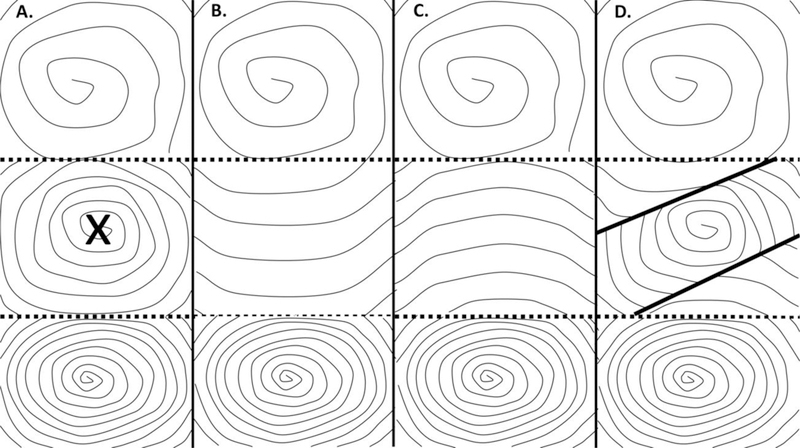
Diagram showing how ablation lesions affect local cycle length for activation, based on timing considerations as addressed by the cellular automaton. A. rectangular (patch) ablation lesions, marked with an x. This will eliminate the center rotational driver. B. The middle region is now activated at a slower rate by the top driver. C. Alternatively, the middle region is now activated at a slower rate by the lower driver with 2:1 conduction alternans into the center region. D. In this panel, simulated linear ablation (thick lines) are applied in proximity to the center rotational driver. Areas then shielded from the center driver arethen driven at a slower rate by the drivers at top and bottom of the panel.
In recent work with this automata, the contribution of simulated APD dispersion, or variation in refractory period, to AF onset and maintenance has been shown and it is similar to what is found in an animal model of AF [28]. In Figure 5, S1-S2 stimuli in a substrate lacking simulated fibrosis but with a refractory period gradient is shown (panel A: refractory period gradient, B-C: propagation of the S1-S2 stimulus wavefronts. However, fibrillatory activity does not occur. Yet, if a random component is added to the refractory period gradient, analogous to APD dispersion (panel D), then after S1-S2 stimulation there is onset of fibrillatory activity (panels E-F). The timing of the spatially heterogeneous refractory period enables progression of wavefronts around areas of functional block, causing synthetic reentrant pathways. Hence, such work may be useful to suggest timing relationships which may be helpful in understanding actual AF events.
5.
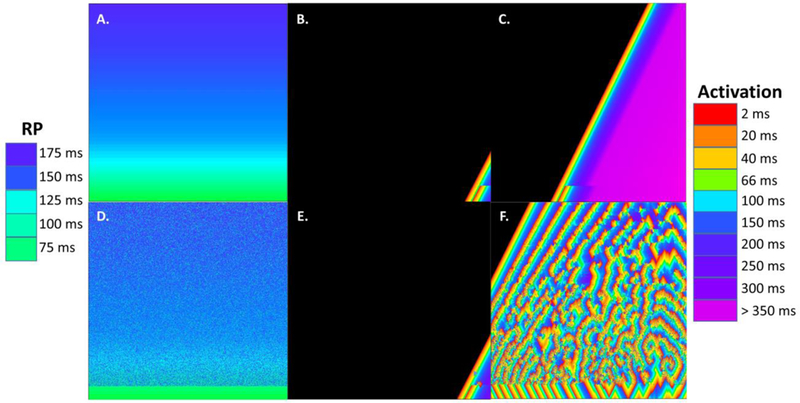
Illustration of the effect of random refractory period changes on simulated fibrillatory activity, cellular automata model. A. The gradient in refractory period ranges from 175 ms (top) to 75 ms (bottom), scale at left. B. An S1-S2 stimuli is applied at the lower right hand corner grid node. The wavefront to the left is S1 and to the right is S2, with the leading edge of each colored red for earliest activation (scale at right). S2 blocks where the refractory period becomes longer than the S1-S2 coupling interval. C. The wavefronts proceed to the opposite side of the grid without occurrence of fibrillatory activity. Since simulated fibrosis was not added, there is nothing to delay the S2 wavefront so that rotational features can form. D. Some grid nodes have been randomly changed in refractory period value as compared with panel A. E. S1-S2 stimuli are imparted to the right hand corner of the grid using the refractory period pattern of panel D. F. Because of the random refractory period imposed in the gradient pattern, functional block occurs at random grid nodes, which in some cases provides sufficient delay so that reentry of the wavefront occurs. The result is a fibrillatory pattern, albeit different from that of Figure 3A.
Summary and Conclusions
In this review, issues concerning the utility of quantitative methods for the analysis of fibrillatory activity as they pertain to AF were discussed. To date, some electrogram time series and frequency measurements have already been found useful to discern and distinguish AF characteristics by quantitation. Furthermore, some events observed in AF can already be simulated by quantitative models. Yet to the present time, many observed AF events are not adequately synthesized by modeling. For example, atrial fiber direction throughout the myocardium is often not included in quantitative modeling, but might be contributive to improve accuracy [89]. With more accurate modeling, it could then be possible to further develop and test hypotheses concerning AF mechanisms. One overwhelming question as yet to be addressed is how AF can progress from the paroxysmal to persistent state, and then to the permanent type in some patients. Perhaps there is an increasing density of drivers, which could enhance activation pattern complexity and contribute to the persistence of arrhythmia. Yet, testing this hypothesis will require more detailed modeling. Successful simulation of observed events by modelling may lead to more efficacious hypothesis testing in the electrophysiology lab, to improve understanding of AF type, and its ability to be terminated via catheter ablation.
Another major goal pertaining to quantitative modeling, as yet unaddressed, is to develop a simulation that can determine, from electrogram time series, the local characteristics of fibrosis, myofibril disarray [90], and APD, which can be important contributors to arrhythmia. This might involve determining whether spectral properties are correlated to optimal candidate driver locations, as has been suggested [91–93]. The process of quantitative modeling and analysis of AF is promising, and is rapidly proceeding. New algorithms are being developed to map microreentrant circuits sustaining fibrillatory activity [94]. Atrial fibrillation can now be distinguished from sinus rhythm using elegant classification algorithms [95]. Machine learning techniques offer potentially new avenues to gain additional insight into the wealth of highly complex spatiotemporal information that is present during fibrillatory activity [96,97]. Nascent, multiscale atrial models have the ability to incorporate high levels of detail of the atrial anatomy, the tissue ultrastructure, and fibrosis distribution [6]. Simulations using such models have demonstrated how an atrial fibrotic substrate and altered atrial electrophysiology might contribute in some cases to the onset and maintenance of AF [6]. Further advancements in quantitative methodology, such as by machine learning [97–99], should be beneficial for better understanding AF mechanisms, the transition from paroxysmal to persistent and permanent AF, when it occurs, and how ablation lesion location and shape, among other procedures, can best be employed to prevent recurrence, minimize laboratory procedure time and invasiveness, and reduce procedural costs and patient morbidity.
Acknowledgments
Dr. Peters acknowledges funding from the British Heart Foundation (RG/16/3/32175 and Centre of Research Excellence), Rosetrees Trust, and the National Institute for Health Research (UK) Biomedical Research Centre.
Footnotes
Conflicts of interest
the authors have no conflicts of interest.
References
- [1].Nattel S Therapeutic implications of atrial fibrillation mechanisms: can mechanistic insights be used to improve AF management? Cardiovasc Res. 2002;54:347–360. [DOI] [PubMed] [Google Scholar]
- [2].Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A, Macle L, Daoud EG, Calkins H, Hall B, Reddy V, Augello G, Reynolds MR, Vinekar C, Liu CY, Berry SM, Berry DA; ThermoCool AF Trial Investigators. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. AMA. 2010;303:333–340. [DOI] [PubMed] [Google Scholar]
- [3].Martins RP, Kaur K, Hwang E, Ramirez RJ, Willis BC, Filgueiras-Rama D, Ennis SR, Takemoto Y, Ponce-Balbuena D, Zarzoso M, O’Connell RP, Musa H, Guerrero-Serna G, Avula UM, Swartz MF, Bhushal S, Deo M, Pandit SV, Berenfeld O, Jalife J. Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation. Circulation 2014;129:1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Camm AJ, Savelieva I, Potpara T, Hindriks G, Pison L, Blömstrom-Lundqvist C. The changing circumstance of atrial fibrillation-progress towards precision medicine. J Intern Med. 2016;279:412–427. [DOI] [PubMed] [Google Scholar]
- [5].Baykaner T, Zaman JAB, Wang PJ, Narayan SM. Ablation of atrial fibrillation drivers. Arrhythm Electrophysiol Rev. 2017;6:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aronis AN, Ali R, Trayanova NA. The role of personalized atrial modeling in understanding atrial fibrillation mechanisms and improving treatment. International Journal of Cardiology 2019. doi: 10.1016/j.ijcard.2019.01.096. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mann I, Sandler B, Linton N, Kanagaratnam P. Drivers of atrial fibrillation: theoretical considerations and practical concerns. Arrhythm Electrophysiol Rev. 2018;7:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yue L, Melnyk P, Gaspo R, Wang Z, Nattel S. Molecular mechanisms underlying ionic remodeling in a dog model of atrial fibrillation. Circ Res. 1999;84:776–784. [DOI] [PubMed] [Google Scholar]
- [9].Vigmond EJ, Ruckdeschel R, Trayanova N. Reentry in a morphologically realistic atrial model. J Cardiovasc Electrophysiol. 2001;12:1046–1054. [DOI] [PubMed] [Google Scholar]
- [10].Wakili R, Voigt N, Kääb S, Dobrev D, Nattel S. Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest. 2011;121:2955–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Christensen K, Manani KA, Peters NS. Simple model for identifying critical regions in atrial fibrillation. Phys Rev Lett. 2015;114:028104–28104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Manani KA, Christensen K, Peters NS, Myocardial architecture and patient variability in clinical patterns of atrial fibrillation, Phys. Rev. 2016;E 94:042401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ciaccio EJ, Biviano AB, Wan EY, Peters NS, Garan H. Development of an automaton model of rotational activity driving atrial fibrillation. Comput Biol Med. 2017;83:166–181. [DOI] [PubMed] [Google Scholar]
- [14].Ciaccio EJ, Peters NS, Garan H. Effects of refractory gradients and ablation on fibrillatory activity. Comput Biol Med. 2018;95:175–187. [DOI] [PubMed] [Google Scholar]
- [15].Ciaccio EJ, Peters NS, Garan H. Use of an automaton model to suggest methods for cessation of intractable fibrillatory activity. Comput Biol Med. 2018;102:357–368. [DOI] [PubMed] [Google Scholar]
- [16].Lin YT, Chang ET, Eatock J, Galla T, Clayton RH. Mechanisms of stochastic onset and termination of atrial fibrillation studied with a cellular automaton model. J R Soc Interface. 2017;14:pii 20160968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ríos-Muñoz GR, Arenal Á, Artés-Rodríguez A. Real-time rotational activity detection in atrial fibrillation. Front Physiol. 2018;9:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu FZ, Liao HT, Liu J, Xue YM, Zhan XZ, Lin WD, Guo HM, Wu SL. Automated ultra–high density mapping in atrial fibrillation hybrid procedure. J Geriatr Cardiol. 2018;15:379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Falk RH. Etiology and complications of atrial fibrillation: insights from pathology studies. Am J Cardiol. 1998;82(8A):10N–17N. [DOI] [PubMed] [Google Scholar]
- [20].Moe GK, Abildskov JA. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am Heart J. 1959;58:59–70. [DOI] [PubMed] [Google Scholar]
- [21].Allessie MA, Bonke FI, Schopman FJ. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. III. The “leading circle” concept: a new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circ Res. 1977;41:9–18. [DOI] [PubMed] [Google Scholar]
- [22].Shivkumar K, Ellenbogen KA, Hummel JD, Miller JM, Steinberg JS. Acute termination of human atrial fibrillation by identification and catheter ablation of localized rotors and sources: first multicenter experience of focal impulse and rotor modulation (FIRM) ablation. J Cardiovasc Electrophysiol. 2012;23:1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Krummen DE, Swarup V, Narayan SM. The role of rotors in atrial fibrillation. J Thorac Dis. 2015;7:142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Misier AR, Opthof T, van Hemel NM, Defauw JJ, de Bakker JM, Janse MJ, van Capelle FJ. Increased dispersion of “refractoriness” in patients with idiopathic paroxysmal atrial fibrillation. J Am Coll Cardiol. 1992;19:1531–1535. [DOI] [PubMed] [Google Scholar]
- [25].Waks JW, Josephson ME. Mechanisms of Atrial fibrillation-Reentry, rotors and reality. Arrhythm Electrophysiol Rev. 2014;3:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. [DOI] [PubMed] [Google Scholar]
- [27].Jalife J, Kaur K. Atrial remodeling, fibrosis, and atrial fibrillation. Trends Cardiovasc Med. 2015;25:475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Avula UM, Abrams J, Katchman A, Zakharov S, Mironov S, Bayne J, Roybal D, Gorti A, Yang L, Iyer V, Waase M, Saluja D, Ciaccio EJ, Garan H, Marks AR, Marx SO, Wan EY. Heterogeneity of the action potential duration is required for sustained atrial fibrillation. JCI Insight. 2019;5:128765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. [DOI] [PubMed] [Google Scholar]
- [30].Harada M, Melka J, Sobue Y, Nattel S. Metabolic considerations in atrial fibrillation-Mechanistic insights and therapeutic opportunities. Circ J. 2017;81:1749–1757. [DOI] [PubMed] [Google Scholar]
- [31].Miragoli M, Glukhov AV. Atrial fibrillation and fibrosis: Beyond the cardiomyocyte centric view. Biomed Res Int. 2015;2015:798768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sohn D, Aronis K, Ashikaga H. Scale-invariant structures of spiral waves. Comput Biol Med. 2019;104:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jalife J, Berenfeld O, Skanes A, Mandapati R. Mechanisms of atrial fibrillation: Mother rotors or multiple daughter wavelets, or both? J Cardiovasc Electrophysiol. 1998;9(8 Suppl):S2–S12. [PubMed] [Google Scholar]
- [34].Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–2053. [DOI] [PubMed] [Google Scholar]
- [35].Hove-Madsen L, Llach A, Bayes-Genís A, Roura S, Rodriguez Font E, Arís A, Cinca J. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation. 2004;110:1358–1363. [DOI] [PubMed] [Google Scholar]
- [36].Bosch RF, Zeng X, Grammer JB, Popovic K, Mewis C, Kühlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res. 1999;44:121–131. [DOI] [PubMed] [Google Scholar]
- [37].Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. [DOI] [PubMed] [Google Scholar]
- [38].Jaïs P, Haïssaguerre M, Shah DC, Chouairi S, Gencel L, Hocini M, Clémenty J. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 1997;95:572–576. [DOI] [PubMed] [Google Scholar]
- [39].Guillem MS, Climent AM, Rodrigo M, Fernández-Avilés F, Atienza F, Berenfeld O. Presence and stability of rotors in atrial fibrillation: evidence and therapeutic implications. Cardiovasc Res. 2016;109:480–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hansen BJ, Zhao J, Csepe TA, Moore BT, Li N, Jayne LA, Kalyanasundaram A, Lim P, Bratasz A, Powell KA, Simonetti OP, Higgins RS, Kilic A, Mohler PJ, Janssen PM, Weiss R, Hummel JD, Fedorov VV. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J. 2015;36:2390–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gianni C, Mohanty S, Trivedi C, Di Biase L, Natale A. Novel concepts and approaches in ablation of atrial fibrillation: the role of non-pulmonary vein triggers. Europace 2018;20:1566–1576 [DOI] [PubMed] [Google Scholar]
- [42].Salinet JL, Tuan JH, Sandilands AJ, Stafford PJ, Schlindwein FS, Ng GA. Distinctive patterns of dominant frequency trajectory behavior in drug-refractory persistent atrial fibrillation: preliminary characterization of spatiotemporal instability. J Cardiovasc Electrophysiol. 2014;25:371–379. [DOI] [PubMed] [Google Scholar]
- [43].Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res. 2014;114:1483–1499. [DOI] [PubMed] [Google Scholar]
- [44].Ciaccio EJ, Biviano AB, Iyer V, Garan H. Trends in atrial flutter and atrial tachycardia research. Informatics in Medicine Unlocked 2017;7:14–22. [Google Scholar]
- [45].Santangeli P, Di Biase L, Al-Ahmad A, Horton R, Burkhardt JD, Sanchez JE, Bai R, Pump A, Mohanty S, Natale A. Ablation for atrial fibrillation: termination of atrial fibrillation is not the end point. Card Electrophysiol Clin. 2012;4:343–352. [DOI] [PubMed] [Google Scholar]
- [46].de Bakker JM, Wittkampf FH. The pathophysiologic basis of fractionated and complex electrograms and the impact of recording techniques on their detection and interpretation. Circ Arrhythm Electrophysiol. 2010;3:204–213. [DOI] [PubMed] [Google Scholar]
- [47].Beheshti M, Magtibay K, Massé S, Porta-Sanchez A, Haldar S, Bhaskaran A, Nayyar S, Glover B, Deno DC, Vigmond EJ, Nanthakumar K. Determinants of atrial bipolar voltage: Inter electrode distance and wavefront angle. Comput Biol Med. 2018;102:449–457. [DOI] [PubMed] [Google Scholar]
- [48].Gojraty S, Lavi N, Valles E, Kim SJ, Michele J, Gerstenfeld EP. Dominant frequency mapping of atrial fibrillation: comparison of contact and noncontact approaches. J Cardiovasc Electrophysiol. 2009;20:997–1004. [DOI] [PubMed] [Google Scholar]
- [49].Oesterlein T, Frisch D, Loewe A, Seemann G, Schmitt C, Dössel O, Luik A. Basket-type catheters: Diagnostic pitfalls caused by deformation and limited coverage. Biomed Res Int. 2016;2016:5340574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mantziari L, Butcher C, Kontogeorgis A, Panikker S, Roy K, Markides V, Wong T. utility of a novel rapid high-resolution mapping system in the catheter ablation of arrhythmias: An initial human experience of mapping the atria and the left ventricle. JACC Clin Electrophysiol. 2015;1:411–420. [DOI] [PubMed] [Google Scholar]
- [51].Rodrigo M, Guillem MS, Climent AM, Pedrón-Torrecilla J, Liberos A, Millet J, Fernández-Avilés F, Atienza F, Berenfeld O. Body surface localization of left and right atrial high-frequency rotors in atrial fibrillation patients: a clinical-computational study. Heart Rhythm 2014;11:1584–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yamashita S, Shah AJ, Mahida S, Sellal JM, Berte B, Hooks D, Frontera A, Jefairi NA, Wielandts JY, Lim HS, Amraoui S, Denis A, Derval N, Sacher F, Cochet H3, Hocini M, Jaïs P, Haïssaguerre M. Body Surface Mapping to Guide Atrial Fibrillation Ablation. Arrhythm Electrophysiol Rev. 2015;4:172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Guillem MS, Climent AM, Millet J, Arenal Á, Fernández-Avilés F, Jalife J, Atienza F, Berenfeld O. Noninvasive localization of maximal frequency sites of atrial fibrillation by body surface potential mapping. Circ Arrhythm Electrophysiol. 2013;6:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].McGillivray MF, Cheng W, Peters NS, Christensen K Machine learning methods for locating reentrant drivers from electrograms in a model of atrial fibrillation. Roy. Soc. Open Sci. 2018;5:172434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Eckstein J, Zeemering S, Linz D, Maesen B, Verheule S, van Hunnik A, Crijns H, Allessie MA, Schotten U. Transmural conduction is the predominant mechanism of breakthrough during atrial fibrillation: evidence from simultaneous endo-epicardial high-density activation mapping. Circ Arrhythm Electrophysiol. 2013;6:334–341. [DOI] [PubMed] [Google Scholar]
- [56].Alonso S, dos Santos RW, Bär M. Reentry and ectopic pacemakers emerge in a three-dimensional model for a slab of cardiac tissue with diffuse microfibrosis near the percolation threshold. PloS One 2016;11:e0166972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Song JS, Wi J, Lee HJ, Hwang M, Lim B, Kim TH, Uhm JS, Joung B, Lee MH, Seo JW, Pak HN. Role of atrial wall thickness in wave-dynamics of atrial fibrillation. PLoS One. 2017. August 21;12(8):e0182174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gray RA, Pertsov AM, Jalife J. Incomplete reentry and epicardial breakthrough patterns during atrial fibrillation in the sheep heart. Circulation. 1996;94:2649–2661. [DOI] [PubMed] [Google Scholar]
- [59].Gutbrod SR, Walton R, Gilbert S, Meillet V, Jaïs P, Hocini M, Haïssaguerre M, Dubois R, Bernus O, Efimov IR. Quantification of the transmural dynamics of atrial fibrillation by simultaneous endocardial and epicardial optical mapping in an acute sheep model. Circ Arrhythm Electrophysiol. 2015;8:456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Nattel S, Xiong F, Aguilar M. Demystifying rotors and their place in clinical translation of atrial fibrillation mechanisms. Nat. Rev. Cardiol. 2017;14:509–520. [DOI] [PubMed] [Google Scholar]
- [61].Yamazaki M, Mironov S, Taravant C, Brec J, Vaquero LM, Bandaru K, Avula UM, Honjo H, Kodama I, Berenfeld O, Kalifa J. Heterogeneous atrial wall thickness and stretch promote scroll waves anchoring during atrial fibrillation. Cardiovasc Res. 2012;94:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lin YJ, Tai CT, Kao T, Chang SL, Wongcharoen W, Lo LW, Tuan TC, Udyavar AR, Chen YJ, Higa S, Ueng KC, Chen SA. Consistency of complex fractionated atrial electrograms during atrial fibrillation. Heart Rhythm 2008;5:406–412. [DOI] [PubMed] [Google Scholar]
- [63].Salinet JL, Masca N, Stafford PJ, Ng GA, Schlindwein FS. Three-dimensional dominant frequency mapping using autoregressive spectral analysis of atrial electrograms of patients in persistent atrial fibrillation. Biomed Eng Online. 2016;15:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ciaccio EJ, Biviano AB, Garan H. Computational method for high resolution spectral analysis of fractionated atrial electrograms. Comput Biol Med. 2013;43:1573–1582. [DOI] [PubMed] [Google Scholar]
- [65].Ng J, Kadish AH, Goldberger JJ. Technical considerations for dominant frequency analysis. J Cardiovasc Electrophysiol. 2007;18:757–764. [DOI] [PubMed] [Google Scholar]
- [66].Habel N, Znojkiewicz P, Thompson N, Müller JG, Mason B, Calame J, Calame S, Sharma S, Mirchandani G, Janks D, Bates J, Noori A, Karnbach A, Lustgarten DL, Sobel BE, Spector P.The temporal variability of dominant frequency and complex fractionated atrial electrograms constrains the validity of sequential mapping in human atrial fibrillation. Heart Rhythm. 2010;7:586–593. [DOI] [PubMed] [Google Scholar]
- [67].Bollmann A, Sonne K, Esperer HD, Toepffer I, Langberg JJ, Klein HU. Non-invasive assessment of fibrillatory activity in patients with paroxysmal and persistent atrial fibrillation using the Holter ECG. Cardiovasc Res. 1999;44:60–66. [DOI] [PubMed] [Google Scholar]
- [68].Ciaccio EJ, Biviano AB, Iyer V, Garan H. Differences in continuous spectra of fractionated electrograms in paroxysmal versus persistent atrial fibrillation. Comput Biol Med. 2016;76:50–59. [DOI] [PubMed] [Google Scholar]
- [69].Atienza F, Calvo D, Almendral J, Zlochiver S, Grzeda KR, Martínez-Alzamora N, González-Torrecilla E, Arenal A, Fernández-Avilés F, Berenfeld O. Mechanisms of fractionated electrograms formation in the posterior left atrium during paroxysmal atrial fibrillation in humans. J Am Coll Cardiol. 2011;57:1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ciaccio EJ, Biviano AB, Garan H. The dominant morphology of fractionated atrial electrograms has greater temporal stability in persistent as compared with paroxysmal atrial fibrillation. Comput Biol Med. 2013;43:2127–2135. [DOI] [PubMed] [Google Scholar]
- [71].Ciaccio EJ, Biviano AB, Whang W, Vest JA, Gambhir A, Einstein AJ, Garan H. Differences in repeating patterns of complex fractionated left atrial electrograms in longstanding persistent atrial fibrillation as compared with paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Cochet H, Dubois R, Yamashita S, Al Jefairi N, Berte B, Sellal JM, Hooks D, Frontera A, Amraoui S, Zemoura A, Denis A, Derval N, Sacher F, Corneloup O, Latrabe V, Clément-Guinaudeau S, Relan J, Zahid S, Boyle PM, Trayanova NA, Bernus O, Montaudon M, Laurent F, Hocini M, Haïssaguerre M, Jaïs P. Relationship between fibrosis detected on late gadolinium-enhanced cardiac magnetic resonance and re-entrant activity assessed with electrocardiographic imaging in human persistent atrial fibrillation. JACC Clin Electrophysiol. 2018;4:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Vigmond E, Pashaei A, Amraoui S, Cochet H, Hassaguerre M. Percolation as a mechanism to explain atrial fractionated electrograms and reentry in a fibrosis model based on imaging data. Heart Rhythm 2016;13:1536–1543. [DOI] [PubMed] [Google Scholar]
- [74].Varela M, Colman MA, Hancox JC, Aslanidi OV. Atrial heterogeneity generates re-entrant substrate during atrial fibrillation and anti-arrhythmic drug action: Mechanistic insights from canine atrial models. PLoS Comput Biol. 2016;12:e1005245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Saha M, Roney CH, Bayer JD2, Meo M, Cochet H, Dubois R, Vigmond EJ. Wavelength and fibrosis affect phase singularity locations during atrial fibrillation. Front Physiol. 2018;9:1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Rostock T, Rotter M, Sanders P, Takahashi Y, Jaïs P, Hocini M, Hsu LF, Sacher F, Clémenty J, Haïssaguerre M. High-density activation mapping of fractionated electrograms in the atria of patients with paroxysmal atrial fibrillation. Heart Rhythm 2006;3:27–34. [DOI] [PubMed] [Google Scholar]
- [77].Hunter RJ, Diab I, Tayebjee M, Richmond L, Sporton S, Earley MJ, Schilling RJ. Characterization of fractionated atrial electrograms critical for maintenance of atrial fibrillation: a randomized, controlled trial of ablation strategies (the CFAE AF trial). Circ Arrhythm Electrophysiol. 2011;4:622–639. [DOI] [PubMed] [Google Scholar]
- [78].Roney CH, Cantwell CD, Qureshi NA, Chowdhury RA, Dupont E, Lim PB, Vigmond EJ, Tweedy JH, Ng FS, Peters NS. Rotor Tracking Using Phase of Electrograms Recorded During Atrial Fibrillation. Ann Biomed Eng. 2017. April;45(4):910–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Vijayakumar R, Vasireddi SK, Cuculich PS, Faddis MN, Rudy Y. Methodology Considerations in Phase Mapping of Human Cardiac Arrhythmias. Circ Arrhythm Electrophysiol 2016;9:e004409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kuklik P, Zeemering S, van Hunnik A, Maesen B, Pison L, Lau DH, Maessen J, Podziemski P, Meyer C, Schaffer B, Crijns H, Willems S, Schotten U. Identification of Rotors during Human Atrial Fibrillation Using Contact Mapping and Phase Singularity Detection: Technical Considerations. IEEE Trans Biomed Eng. 2017;64:310–318. [DOI] [PubMed] [Google Scholar]
- [81].Ciaccio EJ, Biviano AB, Whang W, Gambhir A, Garan H. Spectral profiles of complex fractionated atrial electrograms are different in longstanding and acute onset atrial fibrillation atrial electrogram spectra. J Cardiovasc Electrophysiol. 2012;23:971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Brundel BJ, Henning RH, Kampinga HH, Van Gelder IC, Crijns HJ. Molecular mechanisms of remodeling in human atrial fibrillation. Cardiovasc Res. 2002;54:315–324. [DOI] [PubMed] [Google Scholar]
- [83].Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XHT, Nattel S, Dobrev D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation. 2014;129:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Yue L, Feng J, Gaspo R, Li GR, Wang Z, Nattel S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res. 1997;81:512–525. [DOI] [PubMed] [Google Scholar]
- [85].Courtemanche M1, Ramirez RJ, Nattel S. Ionic targets for drug therapy and atrial fibrillation-induced electrical remodeling: insights from a mathematical model. Cardiovasc Res. 1999;42:477–489. [DOI] [PubMed] [Google Scholar]
- [86].Nishida K1, Michael G, Dobrev D, Nattel S. Animal models for atrial fibrillation: clinical insights and scientific opportunities. Europace. 2010;12:160–172. [DOI] [PubMed] [Google Scholar]
- [87].Haïssaguerre M, Sanders P, Hocini M, Hsu LF, Shah DC, Scavée C, Takahashi Y, Rotter M, Pasquié JL, Garrigue S, Clémenty J, Jaïs P. Changes in atrial fibrillation cycle length and inducibility during catheter ablation and their relation to outcome. Circulation. 2004;109:3007–3013. [DOI] [PubMed] [Google Scholar]
- [88].Haïssaguerre M, Sanders P, Hocini M, Takahashi Y, Rotter M, Sacher F, Rostock T, Hsu LF, Bordachar P, Reuter S, Roudaut R, Clémenty J, Jaïs P. Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J Cardiovasc Electrophysiol. 2005;16:1125–1137. [DOI] [PubMed] [Google Scholar]
- [89].Roney CH, Whitaker J, Sim I, O’Neill L, Mukherjee RK, Razeghi O, Vigmond EJ, Wright M, O’Neill MD, Williams SE, Niederer SA. A technique for measuring anisotropy in atrial conduction to estimate conduction velocity and atrial fibre direction. Comput Biol Med. 2019;104:278–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hwang C, Karagueuzian HS, Chen PS. Idiopathic paroxysmal atrial fibrillation induced by a focal discharge mechanism in the left superior pulmonary vein: possible roles of the ligament of Marshall. J Cardiovasc Electrophysiol. 1999;10:636–648. [DOI] [PubMed] [Google Scholar]
- [91].Ng J, Kadish AH, Goldberger JJ. Effect of electrogram characteristics on the relationship of dominant frequency to atrial activation rate in atrial fibrillation. Heart Rhythm 2006;3:1295–1305. [DOI] [PubMed] [Google Scholar]
- [92].Takahashi Y, Sanders P, Jaïs P, Hocini M, Dubois R, Rotter M, Rostock T, Nalliah CJ, Sacher F, Clémenty J, Haïssaguerre M. Organization of frequency spectra of atrial fibrillation: relevance to radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2006;17:382–388. [DOI] [PubMed] [Google Scholar]
- [93].Paliakaitė B, Petrėnas A, Henriksson M, Skibarkienė J, Kubilius R, Sörnmo L, Marozas V. Atrial fibrillation frequency tracking in ambulatory ECG signals: The significance of signal quality assessment. Comput Biol Med. 2018;102:227–233. [DOI] [PubMed] [Google Scholar]
- [94].Handa BS, Roney CH, Houston C, Qureshi NA, Li X, Pitcher DS, Chowdhury RA, Lim PB, Dupont E, Niederer SA, Cantwell CD, Peters NS, Ng FS. Analytical approaches for myocardial fibrillation signals. Comput Biol Med. 2018;102:315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kruger GH, Latchamsetty R, Langhals NB, Yokokawa M, Chugh A, Morady F, Oral H, Berenfeld O. Bimodal classification algorithm for atrial fibrillation detection from m-health ECG recordings. Comput Biol Med. 2019;104:310–318. [DOI] [PubMed] [Google Scholar]
- [96].Cantwell CD, Mohamied Y, Tzortzis KN, Garasto S, Houston C, Chowdhury RA, Ng FS, Bharath AA, Peters NS. Rethinking multiscale cardiac electrophysiology with machine learning and predictive modelling. Comput Biol Med. 2019;104:339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Hagiwara Y, Fujita H, Oh SL, Tan JH, Tan RS, Ciaccio EJ, Acharya UR. Computer-aided diagnosis of atrial fibrillation based on ECG Signals: A review. Information Sciences 2018;467:99–114. [Google Scholar]
- [98].Acharya UR, Faust O, Ciaccio EJ, Wei JKE, Lih OS, San TR, Garan H. Application of nonlinear methods to discriminate fractionated electrograms in paroxysmal versus persistent atrial fibrillation. Computer Methods and Programs in Biomedicine 2019;175:163–178. [DOI] [PubMed] [Google Scholar]
- [99].Yildirim O, Baloglu UB, Tan RS, Ciaccio EJ, Acharya UR. A new approach for arrhythmia classification using deep coded features and LSTM Networks. Computer Methods and Programs in Biomedicine 2019. (in press) DOI: 10.1016/j.cmpb.2019.05.004. [DOI] [PubMed] [Google Scholar]


