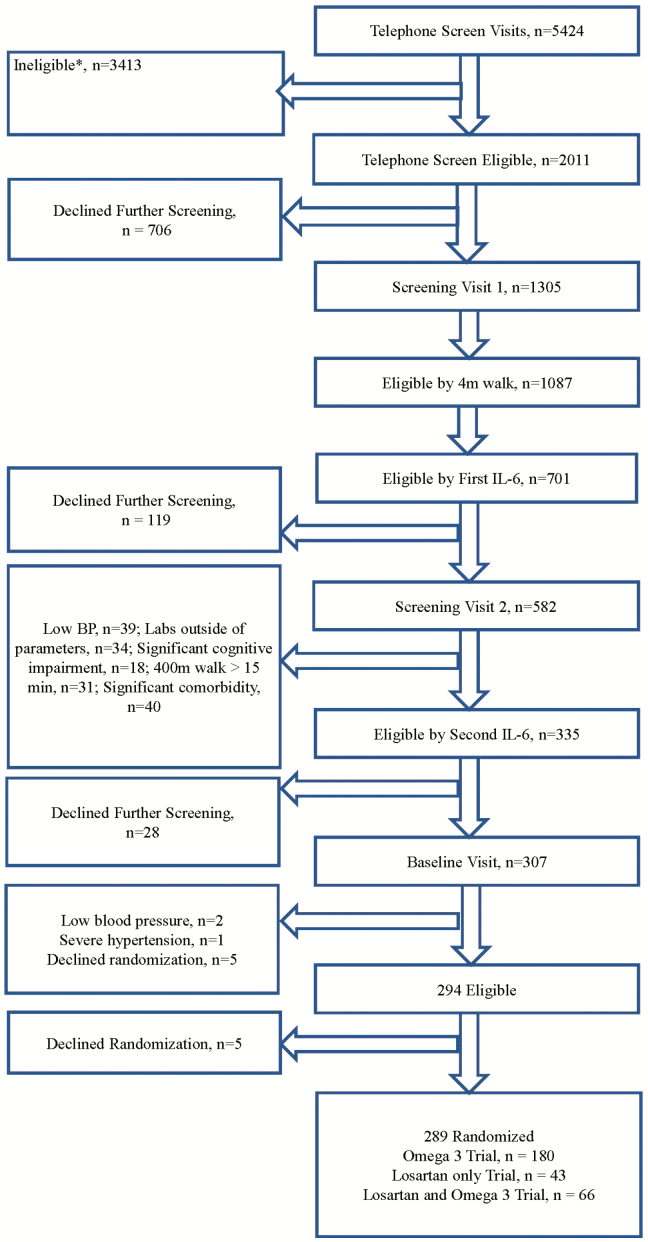
Figure 1.
Consort flow chart. Potential participants could be ineligible for more than one reason. *No self-reported difficulty walking ¼ mile or climbing steps, n = 1,183. *Current use of ACEI or ARB for those ineligible for both Losartan and Fish Oil trials, n = 623. *Currently taking omega-3/fish oil for those ineligible for both Losartan and Fish Oil trials, n = 344. *Usually use a walker to get around, n = 246. * Known/active inflammatory disease, n = 230. *Prior or current atrial fibrillation, for those ineligible for both Losartan and *Unable to walk one block, n = 204. *Current smoker, n = 185. *Currently receiving physical therapy for gait, balance, n = 123. *Use of potassium sparing diuretics for those ineligible for both Losartan and Fish Oil trials, n = 96. *Lives outside area/relocations, n = 86. *Neurologic conditions, impaired mobility, n = 75. *Allergy to fish, shell fish, and/or fish oil for those ineligible for both Losartan and Fish Oil trials, n = 70. *Acute Infection, n = 66. *>14 alcohol drinks/week, n = 53. *Severe pulmonary disease, n = 47. *Participation in another intervention study, n = 47. *Consumed more than two servings per week of fish in the past year for those ineligible for both Losartan and Fish Oil trials, n = 44. *Age <70, n = 41.