Scheme 2.
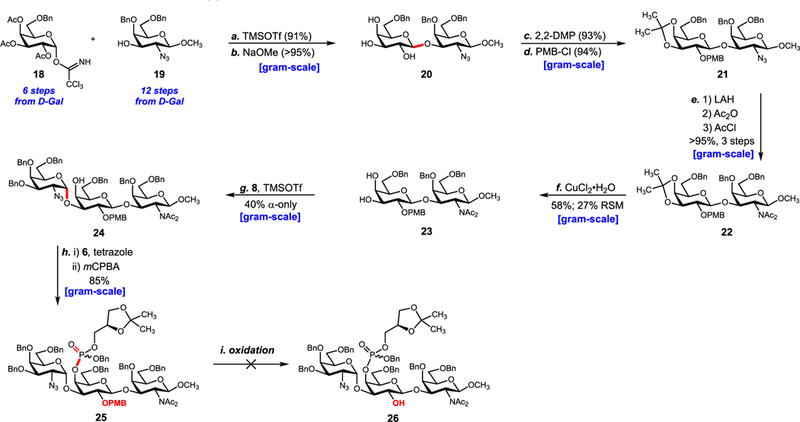
Second-Generation Approacha aReagents and conditions: (a) TMSOTf (0.15 equiv), CH2Cl2, 0 °C → 25 °C, 1 h, β-only, 91%; (b) NaOCH3, CH3OH, 25 °C, 2 h, > 95%; (c) 2,2-DMP, p-TsOH, CH2Cl2, 25 °C, 0.5 h, 93%; (d) PMBCl (1.2 equiv), NaH (2.0 equiv), DMF, 0 °C → 25 °C, 1 h, 94%; (e) LiAlH4, THF, 0 °C → 25 °C, 1 h then Ac2O, pyr., DMAP, 0 °C → 25 °C, 2 h then AcCl, DIPEA, 2:3 CH2Cl2/CH3CN, μwave 85 °C, 3 h, >95% over three steps; (f) CuCl2·H2O, CH3CN, 0 °C → 25 °C, 3 h, 58% with 27% RSM; (g) 8 (2.5 equiv), TMSOTf (0.15 equiv), CH2Cl2, −78 °C→ −40 °C, 2.5 h, α-only, 40%; (h) 6 (1.5 equiv), 1H-tetrazole (1.5 equiv), CH3CN, 0 °C → 25 °C, 0.5 h then mCPBA (1.5 equiv), CH2Cl2, −78 °C → 0 °C, 2 h, 85%.
