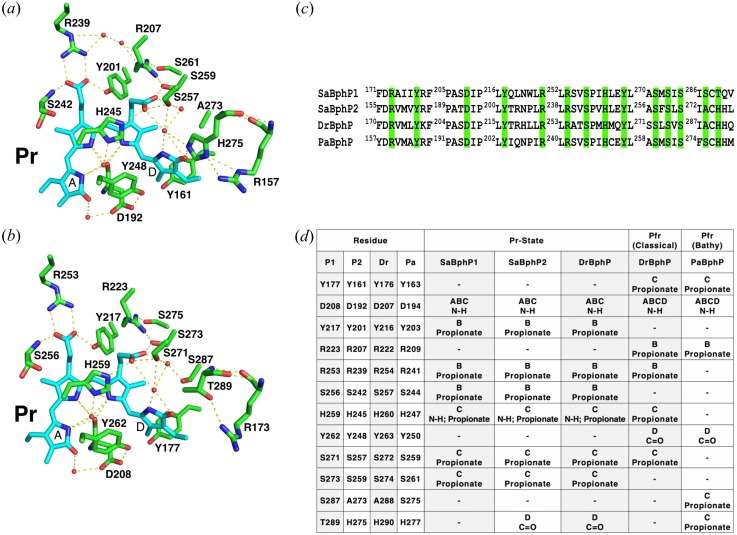
FIG. 2.
Hydrogen-bonding network of the conserved amino acids and neighboring water molecules stabilizing BV chromophore in the Pr state of SaBphP2 wild-type (a) and SaBphP1 wild-type (b). Hydrogen bonds are marked with dashed lines, and amino acids located in the GAF domain are highlighted in green. (c) Partial protein sequence alignment highlighting conserved amino acids stabilizing propionate side chains of BV chromophore (in cyan) as well as A-D pyrrole rings. (d) Table with conserved amino acids from SaBphP1, SaBphP2, DrBphP, and bathy PaBphP with specific BV interactions as identified in the Pr and Pfr states.