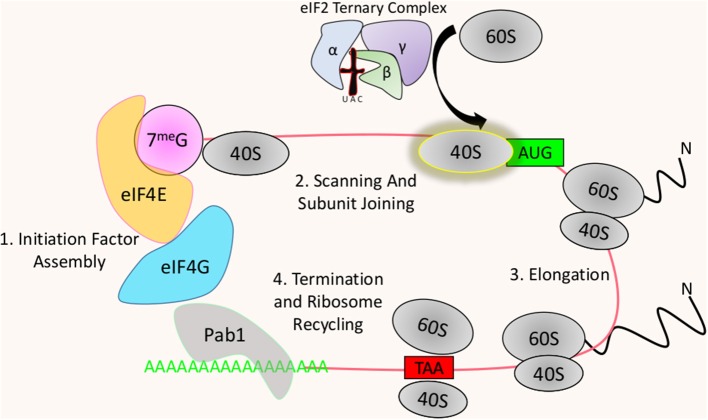
Figure 1.
Brief overview of translation: (1) Translation begins with the recruitment of the cap-binding complex to the 5′ end of the mRNA allowing interaction with the Poly-A binding protein (Pab1)-associated 3′ poly-A tail. All Eukaryotic mRNAs are capped by a modified nucleotide at the 5′ end, which is typically a N7-methylated guanosine. eIF4E recognizes the cap and recruits the scaffolding initiation factor eIF4G. eIF4G itself recruits a suite of initiation factors including Pab1, which binds to the poly-a tail. The interaction between eIF4G and Pab1 bridges the 5′ and 3′ ends of the mRNA forming a “closed loop” structure that is thought to stimulate translation. (2) At this point the 40S subunit is recruited to the 5′ end of the mRNA where it begins scanning the transcript until reaching a start codon. The corresponding initiator tRNA is delivered to the 40S subunit at the start site by the eIF2 ternary complex, allowing for the joining of the 60S subunit (3). Following ribosome assembly, translation elongation begins with the recruitment of tRNAs charged with their respective amino acids. (4) Elongation ends when the ribosome recognizes a stop codon, where no corresponding tRNA exists, resulting in the release of the produced polypeptide and the dissociation of the 60S and 40S subunits thereby allowing their use for further rounds of translation.