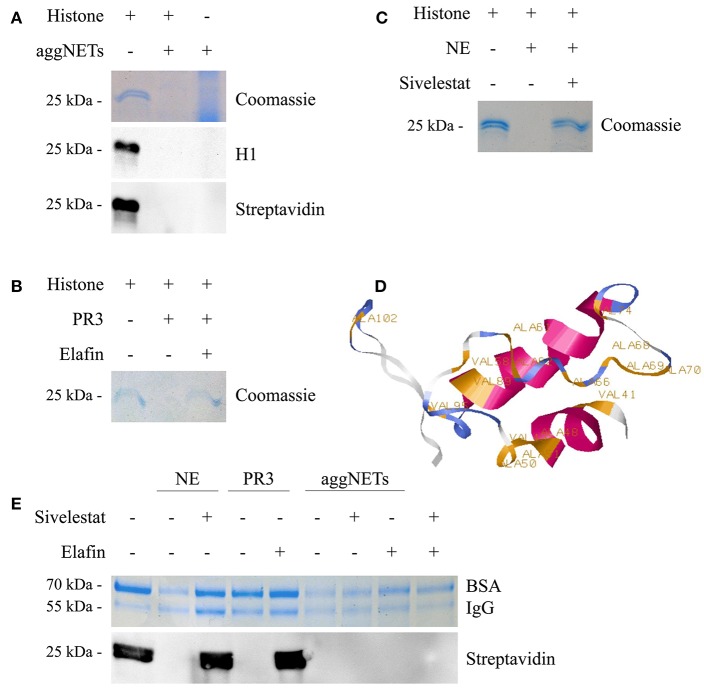
Figure 1.
aggNETs degrade histones. (A) Histones incubated with aggNETs are degraded as seen in Coomassie and staining and anti-histone H1 Blot. The biotinylationed histones are not bound by the aggNETs. (B) Proteinase3 (PR3) degrades histones. This degradation is inhibited by Elafin as seen in the Coomassie staining. (C) Neutrophil Elastase (NE) degrades histones, specifically inhibited by Sivelestat as shown in the Coomassie staining. (D) SWISS-MODEL of histone H1 (amino acids 39–119) with the cleavage sites for NE as predicted by ExPASy PeptideCutter. (E) NE and aggNETs favor histone over bovine serum albumin (BSA) and human Immunoglobulin G (IgG) for degradation; whereas PR3 can only degrade histones. Degradation of biotinylated histones by aggNETs is not inhibited by Sivelestat or Elafin or a combination of both as seen by the detection of Streptavidin HRP in Western Blot analysis. SDS-PAGE, Western Blot Analysis and Coomassie staining in (A–C) were performed after incubation of the samples for 24 h at 37°C. For (E) the incubation time was 8 h at 37°C. All images shown are representative images of at least three independent experiments. The full-sized images are shown in Figures S1A–D. The successful formation of an aggNET is shown in the macrophotographs in Figure S1E in bright-field and under UV after staining with propidium iodide.