Table 1.
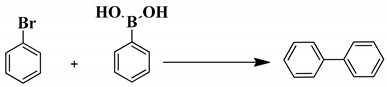
Optimization of the reaction conditions for the Suzuki reaction of bromobenzene with phenyl boronic acid catalyzed by m-f-MWCNTs@chitosan NHC-Pd nanocomposite.
| Entry | Amount of Catalyst (mol%) | T (°C) | Solvent | Time (h) |
Conversion (%) |
Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 0.1 | 80 | H2O | 5 | 80 | 75 |
| 2 | 0.5 | 80 | H2O | 5 | 90 | 84 |
| 3 | 0.5 | 80 | H2O-EtOH | 5 | 100 | 97 |
| 4 | 0.3 | 80 | H2O-EtOH | 5 | 98 | 94 |
| 5 | 0.1 | 80 | H2O-EtOH | 5 | 97 | 94 |
| 6 | 0.1 | 50 | H2O-EtOH | 3 | 97 | 94 |
| 7 | 0.08 | 50 | H2O-EtOH | 3 | 85 | 80 |
Reaction conditions: Phenyl boronic acid (1.2 mmol), bromobenzene (1 mmol), K2CO3 (3 mmol), m-f-MWCNTs@chitosan NHC-Pd nanocomposite as a catalyst, Solvent (H2O-EtOH: 1:1 (6 mL)), calculated by GC.
