Figure 3.
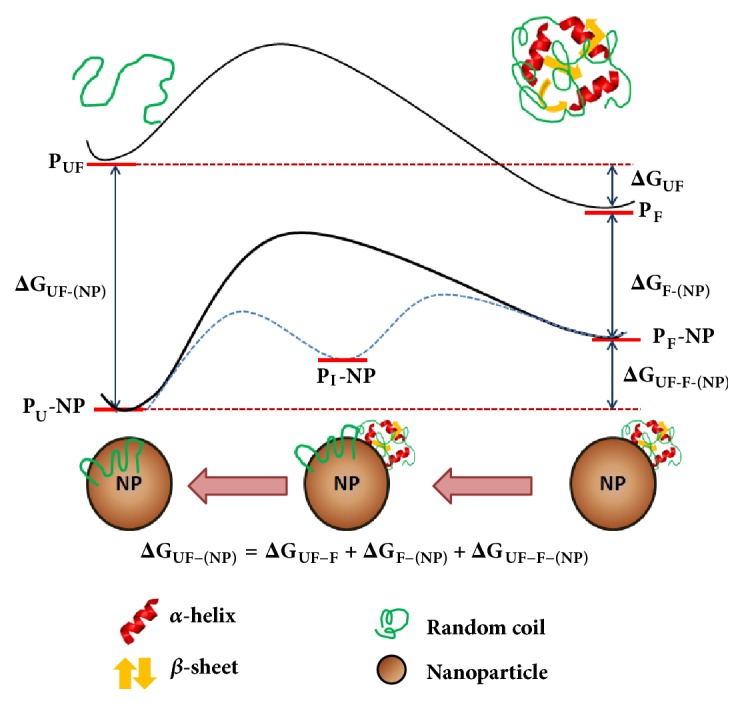
Free energy profiles of the protein–NP interaction. A schematic representation of the energy profiles of the protein–NP interaction and its influence on the folding of the protein. From the thermodynamics point of view, the native or folded state of protein (PF) is only marginally more stable than the unfolded state (PUF) physiologically. The binding energy of PUF with a NP is usually larger than that of PF. Correspondingly, the PUF–(NP) complex is usually more stable than the PF–NP complex. From the equation in the diagram it can be shown that larger free energy change of the binding between the folded protein and the NP ( ΔG F-NP ) means a smaller free energy change of the unfolding of the bound protein on the NP surface ( ΔG UF-F-NP ).
