Abstract
As sources of reactive halogens, snowpacks in sea ice regions control the oxidative capacity of the Arctic atmosphere. However, measurements of snowpack halide concentrations remain sparse, particularly in the high Arctic, limiting our understanding of and ability to parameterize snowpack participation in tropospheric halogen chemistry. To address this gap, we measured concentrations of chloride, bromide, and sodium in snow samples collected during polar spring above remote multi-year sea ice (MYI) and first-year sea ice (FYI) north of Greenland and Alaska, as well as in the central Arctic, and compared these measurements to a larger dataset collected in the Alaskan coastal Arctic by Krnavek et al. (2012). Regardless of sea ice region, these surface snow samples generally featured lower salinities, compared to coastal snow. Surface snow in FYI regions was typically enriched in bromide and chloride compared to seawater, indicating snowpack deposition of bromine and chlorine-containing trace gases and an ability of the snowpack to participate further in bromine and chlorine activation processes. In contrast, surface snow in MYI regions was more often depleted in bromide, indicating it served as a source of bromine-containing trace gases to the atmosphere prior to sampling. Measurements at various snow depths indicate that the deposition of sea salt aerosols and halogen-containing trace gases to the snowpack surface played a larger role in determining surface snow halide concentrations compared to upward brine migration from sea ice. Calculated enrichment factors for bromide and chloride, relative to sodium, in the MYI snow samples suggests that MYI regions, in addition to FYI regions, have the potential to play an active role in Arctic boundary layer bromine and chlorine chemistry. The ability of MYI regions to participate in springtime atmospheric halogen chemistry should be considered in regional modeling of halogen activation and interpretation of satellite-based tropospheric bromine monoxide column measurements.
Keywords: Arctic, Snow, Sea ice, Bromine, Chlorine, Halogen
Introduction
Snowpack-driven halogen chemistry has a profound impact on atmospheric composition in the polar regions (Simpson et al., 2007b; Abbatt et al., 2012). The release of Br2 from the surface snowpack (Pratt et al., 2013) following polar sunrise leads to boundary layer ozone depletion events (ODEs) (Barrie et al., 1988; Simpson et al., 2007b), as well as mercury deposition events (Schroeder et al., 1998; Steffen et al., 2008). Currently, the Arctic cryosphere is undergoing rapid changes (Bhatt et al., 2014), including declining sea ice extent (Stroeve et al., 2007), as well as thinning snowpacks in the western Arctic Ocean due to delayed sea ice formation (Webster et al., 2014). Due to the coupling between the atmosphere and the cryosphere (Domine and Shepson 2002), these surface changes are expected to impact the production of molecular halogens from the snowpack. Bromine activation, the conversion of condensed phase bromide to atmospheric reactive bromine (e.g. Br, BrO), has been linked to sea ice regions, particularly first year ice (FYI) regions (Wagner and Platt, 1998; Simpson et al., 2007a). FYI regions contain a wide variety of saline ice surfaces (e.g. frost flowers, saline snow, freshly frozen sea ice) (Perovich and Richter-Menge, 1994) that have been previously implicated in bromine activation (Abbatt et al., 2012). However, recent studies show the overlying snowpack, rather than frost flowers or the sea ice surface itself, is primarily responsible for nearsurface bromine activation processes (e.g. Piot and von Glasow, 2008; Pratt et al., 2013; Custard et al., 2017; Raso et al., 2017). In particular, Pratt et al. (2013) showed that snowpack Br2 production depends on snowpack chemical composition rather than purely salinity. Long term observations of bromine monoxide by Burd et al. (2017) show this bromine chemistry does not occur after snow melt onset, but can recur after new snowfall, further pointing to the critical role of the snowpack in this bromine chemistry. The snowpack is also a source of Cl2 and BrCl (Custard et al., 2017), leading to increased chlorine levels in the lower troposphere (Liao et al., 2014; Custard et al., 2016). This atmospheric chlorine chemistry increases the oxidation of hydrocarbons (Jobson et al., 1994), reducing the lifetime of the greenhouse gas methane (Platt et al., 2004). Given the influence of the snowpack on the chemical composition of the overlying atmosphere (Domine and Shepson, 2002), understanding the environmental factors that control snowpack chemical composition, particularly in remote sea ice regions, is crucial.
Halides become incorporated into the snowpack through a variety of mechanisms. Sea spray aerosol (SSA) can nucleate cloud droplets and ice crystals, contributing to snowfall (DeMott et al., 2016). Snow is also expected to scavenge halide-containing aerosols and gas phase halogens during precipitation (Macdonald et al., 2017). However, measurements of Arctic snowfall bromide and chloride concentrations (10−2–102 μM) by Toom-Sauntry and Barrie (2002) suggest these mechanisms do not contribute substantially to snowpack halide concentrations (10−2–106 μM, Krnavek et al. (2012)). As snow accumulates, brine migrates through the interface between snow and saline sea ice into the overlying snowpack (Perovich and Richter-Menge, 1994; Domine et al., 2004). This mechanism of providing salt to the surface snowpack becomes less effective as snowpack depth increases, with an upper limit of brine migration of ≈17 cm above the sea ice observed by Domine et al. (2004). FYI is more saline than multi-year ice (MYI) (Cox and Weeks, 1973), where available brine that can be transported into the snowpack is limited. In addition, snow depth in MYI regions is generally greater than in FYI regions, due to accumulation of snowfall in autumn when sea ice extent is close to an annual minimum (Webster et al., 2014; Blanchard-Wrigglesworth et al., 2015). Thus, this relationship between snow depth and salinity has been suggested to lead to differing abilities of surface snow in FYI and MYI regions to participate in atmospheric bromine chemistry (Abbatt et al., 2012).
Halides can also be added to the surface snowpack by the deposition of SSA (Nilsson and Rannik, 2001; Domine et al., 2004). The open ocean produces SSA through wave-breaking (Lewis and Schwartz, 2004), with emissions expected to increase with declines in sea ice extent (Browse et al., 2014). SSA production also occurs over sea ice leads (Leck et al., 2002; Nilsson et al., 2001), providing a year-round source of SSA (May et al., 2016). This mechanism provides salts to the surface snowpack regardless of snow depth, allowing the surface of deep snowpacks in sea ice regions to still contain halides (Domine et al., 2004). Trace bromine gases (e.g. HBr, HOBr, BrONO2) produced within the snowpack or through multi-phase reactions on aerosol particles are also deposited onto the snowpack surface enhancing snowpack bromide concentrations hundreds of kilometers from initial sources (Simpson et al., 2005). Snowpack reactions also release bromine to the atmosphere from the snowpack through condensed and multiphase reactions with Br− to produce Br2 (Pratt et al., 2013; Raso et al., 2017; Custard et al., 2017). Similarly, trace chlorine gases (e.g. HCl, HOCl, ClONO2) can be deposited on the snowpack surface, increasing chloride (Cl−) concentrations and leading to the production of BrCl and Cl2 (Custard et al., 2017). Macdonald et al. (2017) observed a higher effective deposition of chloride than sodium to the snowpack at Alert, Nunavut, Canada and attributed this to the deposition of trace chlorine gases. Each of these mechanisms lead to differing levels of snowpack halide concentrations and enrichments relative to seawater. Thus, determining snowpack chemical composition in FYI and MYI regions enables elucidation of the sources of halides (e.g. brine or deposition), and the extent to which these regions might participate in atmospheric halogen chemistry.
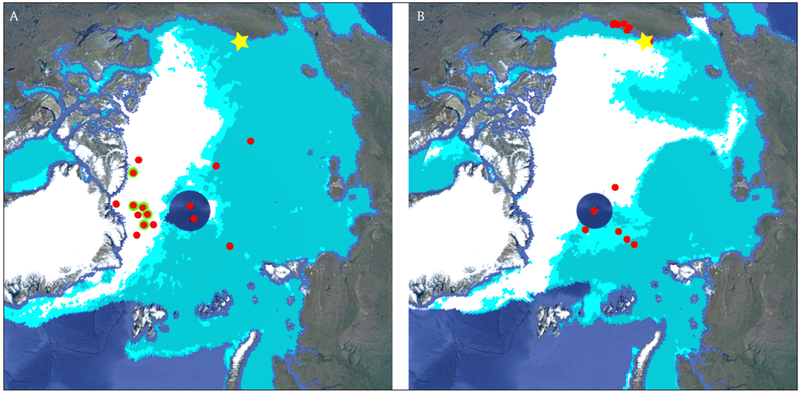
Many studies (Toom-Sauntry and Barrie, 2002; Domine et al., 2004; Douglas and Sturm 2004; Simpson et al., 2005; de Caritat et al., 2005; Krnavek et al., 2012; Jacobi et al., 2012; Pratt et al., 2013; Xu et al., 2016) have examined the halide content of snow in the coastal Arctic. Toom-Sauntry and Barrie (2002) observed bromide in fresh snowfall at Alert, Nunavut, Canada, to increase after polar sunrise and attributed this increase to atmospheric bromine chemistry. In contrast, chloride in snowfall was highest during the winter and did not increase after polar sunrise (Toom-Sauntry and Barrie, 2002). Studies of snowpack composition in northern Alaska (e.g. Simpson et al., 2005; Krnavek et al., 2012; Jacobi et al., 2012) showed coastal snowpack bromide concentrations spanning several orders of magnitude (10−2–10−4 μM), implying modification of bromide content in the snowpack by atmospheric halogen chemistry processes (e.g. Simpson et al., 2005; Krnavek et al., 2012). While snowpack chemical composition has been extensively studied in Arctic coastal regions, our knowledge of available snowpack halide concentrations in more remote Arctic sea ice regions remains limited by a lack of measurements of snowpack chemical composition within remote sea ice regions in the high Arctic. This study uses bulk inorganic ion composition measurements of surface snow samples collected from both MYI and FYI regions in the remote Arctic Ocean (Figure 1) during spring to investigate the potential role of these regions in atmospheric halogen chemistry.
Figure 1:
Surface snow sampling locations across the Arctic Ocean. A) Sampling locations during April and May 2013 (red markers) overlaid on a sea ice map from 17 April 2013. Locations where snow was sampled at multiple depths are highlighted in green. B) Sampling locations from February and April of 2014 (red markers) overlaid on a sea ice map from 14 February 2014. The sea ice maps are derived from Oceansat-2 satellite scatterometry, unavailable over the North Pole. On these maps, white represents multiyear ice (MYI) regions, light blue represents first year ice (FYI) regions, and cyan represents a combination of MYI and FYI regions. The approximate sampling locations from the Krnavek et al. (2012) study are indicated with a yellow star. DOI: https://doi.org/10.1525/elementa.352.f1
Methods
Snow sample collection
Snow samples were collected from locations in both FYI and MYI regions across the Arctic Ocean accessed via aircraft (Figure 1) in April 2013, May 2013, February 2014, and April 2014. Given the significant logistical challenges and cost of conducting fieldwork in the central Arctic, these samples were collected as part of the North Pole Environmental Observatory, Switchyard, and US Interagency Arctic Buoy programs, with snow sampling considered to be an add-on to the program goals, which determined the sampling locations. The dataset consists of 29 snow samples from the surface of the snowpack (top 1–3 cm), with 11 samples being collected over MYI, and 18 collected over thicker (1.5–2 m) FYI. At five sites, vertical profiles consisting of three samples at differing snow depths were taken (Figure S1). Ice type and snow depth were noted at each sampling location, as well as the latitude and longitude coordinates (Table S1). This ice type classification is based on true local observations, rather than the synoptic satellite scatterometry based classifications (Nghiem et al., 2005, 2006, 2007) shown in Figure 1 for visualization purposes. Samples at each site were collected upwind of the aircraft in an area of undisturbed snow, with a polypropylene scoop into polyethylene bags (Whirl-Pak, Nasco), which are sealed with a tear-off top prior to sample collection to prevent contamination. Excess air was then removed, and the sample bag was placed in a second, larger, polyethylene bag. Samples were shipped frozen to the University of Michigan in Ann Arbor, MI where they were kept at −40°C in the dark prior to analysis. The six samples collected from sea ice regions in the central Arctic in 2014 (Figure 1B) melted during transit and were refrozen upon arrival in Ann Arbor.
Given the limited sample size, we discuss these samples in the context of 772 previously collected samples, also analyzed by ion chromotography (IC), from both MYI and FYI sea ice regions north of Alaska during the spring of 2004, 2005, and 2007 (Krnavek et al., 2012). The sea ice regions sampled were off the coast north of Utqiaġvik, AK (71.2906°N, 156.7886°W) and in the vicinity of the 2007 Applied Physics Laboratory Ice Station, approximately 300 km to the north-east of Utqiaġvik.
Ion chromotography (IC)
One day prior to analysis, snow samples were transferred to a refrigerator (≈6°C) and melted. The resulting meltwater was sampled in triplicate using 1 mL disposable syringes equipped with 0.22 μM polyvinylidene fluoride (PVDF) filters. IC analyses (Harris, 2016) were performed using Dionex ICS-1100 and ICS-2100 systems (Thermo Scientific) for cations and anions, respectively, in the melted snow samples. The chromatographs each contained a 200 μL sample loop and a heated conductivity cell (Dionex DS6, Thermo Scientific). Each chromatograph contained a guard column (ICS-1100: Dionex IonPac CG12A-5 μm, 3 × 30 mm, Thermo Scientific; ICS-2100: Dionex IonPac AG18, 4 × 50 mm, Thermo Scientific), analytical column (ICS-1100: Dionex IonPac CS12A-5 μm, 3 × 150 mm, Thermo Scientific; ICS-2100: Dionex IonPac AS18 4 mm, 4 × 250 mm, Thermo Scientific), and suppressor (ICS-1100: Dionex CSRS 500, 4 mm, Thermo Scientific; ICS-2100: Dionex AERS500, 4 mm, Thermo Scientific). A potassium hydroxide (KOH) gradient generated by a Dionex EGC III KOH system (Thermo Scientific) was used as the eluent for the anion IC, and 20 mM methanesulfonic acid was used as eluent for the cation IC. The inorganic ions discussed in this manuscript, and their corresponding detection limits, were sodium (Na+; 6 μM), chloride (Cl−; 0.7 μM), and bromide (Br−; 0.02 μM). Samples that gave initial signals outside of the instrument calibration range were diluted with 18.2 MΩ cm−1 water for final analysis. Measurement errors shown are the standard deviation of triplicate measurements. Concentrations and measurement errors are shown in Table S2 (Peterson and Pratt, 2017). Following IC analysis, samples were analyzed in triplicate for pH with an Accumet AP110 pH probe. pH measurements, location, and snowpack properties for each sample are provided in Table S1 (Peterson and Pratt 2017).
Results and Discussion
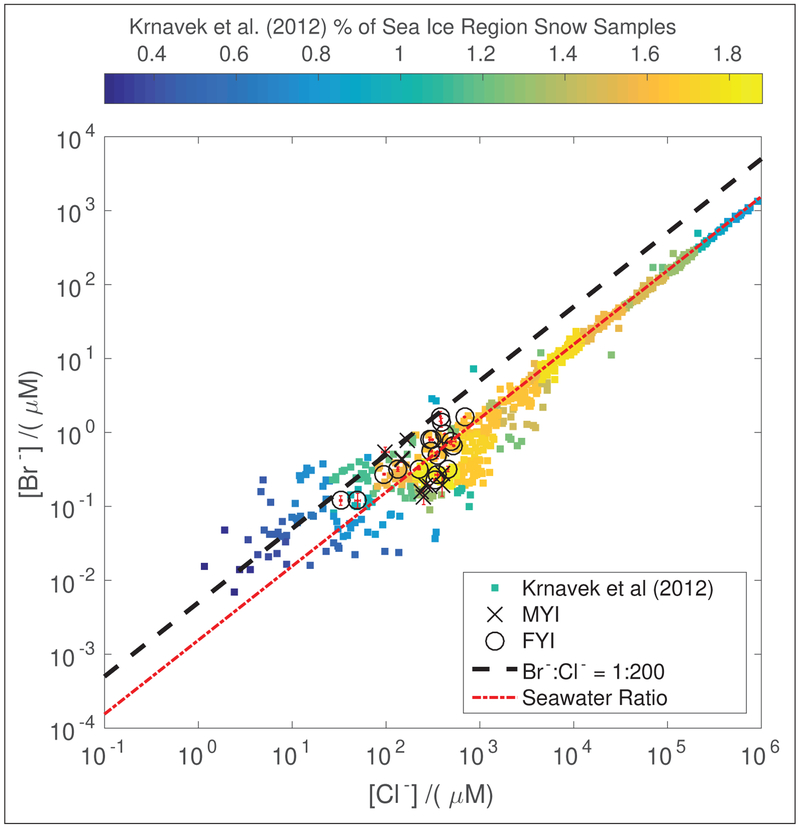
Chloride (Cl−) and bromide (Br−) concentrations for all snow meltwater samples are shown in Figure 2. The samples in this study generally had lower halide concentrations ([X−] < 103 μM) compared to prior studies (e.g. Krnavek et al. (2012), [X] < 106 μM). This is likely due to sampling snow in regions with thicker (>1 m), more consolidated sea ice, which tends to be less saline than snow above thinner sea ice sampled in prior studies (Perovich and Richter-Menge 1994; Krnavek et al., 2012). In the current study, samples from MYI regions had an average [Br−] of 0.36 ± 0.06 μM (standard error of the mean), while samples from FYI regions had an average [Br−] of 0.6 ± 0.1 μM. With respect to chloride, samples from MYI regions had an average [Cl−] of 250 ± 30 μM, while samples from FYI regions had an average [Cl−] of 320 ± 40 μM. While the average bromide and chloride concentrations were higher in surface snow from FYI regions than MYI regions, these differences are not statistically significant at the 95% confidence level for either anion, likely due to the small sample size.
Figure 2:
Snow meltwater bromide (Br−) and chloride (Cl−) concentrations for each sample grouped by the ice region where the sample was collected. Concentration uncertainties are calculated using the standard deviation of triplicate IC measurements and plotted in red. The seawater bromide to chloride mole ratio (Millero et al., 2008) is plotted in red for reference. The black line indicates the approximate minimum ratio of bromide to chloride for efficient snowpack Br2 production in prior studies (Pratt et al., 2013). To provide context for these data, snow sample concentrations in FYI and MYI regions north of Alaska previously presented by Krnavek et al. (2012) are shown. The Krnavek et al. (2012) data are colored by the percentage of observations, with more commonly measured concentrations plotted in yellow and less common concentrations plotted in blue. DOI: https://doi.org/10.1525/elementa.352.f2
Pratt et al. (2013) showed that the ratio of bromide to chloride (Br−/Cl−) plays a role in enabling the production of Br2 from the snowpack, and suggested a Br−/Cl− mole ratio greater than 1:200 as being optimal for Br2 production, based on available coastal tundra and FYI region snow samples. This dependence of Br2 production on bromide enrichment has also been observed in previous laboratory studies (e.g. Huff and Abbatt 2002; Adams et al., 2002; Sjostedt and Abbatt 2008). As shown in Figure 2, Br−/Cl− mole ratios in excess of 1:200 were not commonly observed in either these data or the Krnavek et al. (2012) dataset. Examining the Krnavek et al. (2012) dataset shows ratios in excess of 1:200 occurred ≈15% of the time in MYI regions and less than 5% of the time in FYI regions. Given the prevalence of tropospheric BrO in satellite observations over sea ice regions in the Arctic spring (e.g. Wagner and Platt 1998; Theys et al., 2011; Koo et al., 2012), our measurements suggest less enriched Br−/Cl− ratios than previously suggested by Pratt et al. (2013) regularly enable Br2 production. However, Pratt et al. (2013) did not have available snow samples with acidic pH and enriched Br−/Cl− ratios to test Br2 production. Prior studies suggest enhancements in the Br−/Cl− ratio are more common at low salinity, which is commonly observed in snow samples from coastal tundra and MYI regions (Krnavek et al., 2012), as well as at Summit, Greenland (Dibb et al., 2010). Given the demonstrated effectiveness of less saline coastal tundra snowpacks in producing Br2 (Pratt et al., 2013), it is reasonable to suggest that low salinity snowpacks sampled in this study are similarly effective in producing Br2. The effectiveness of snowpacks in MYI regions as a source of Br2 is supported by the large enhancements in tropospheric BrO columns observed in MYI regions by Peterson et al. (2016).
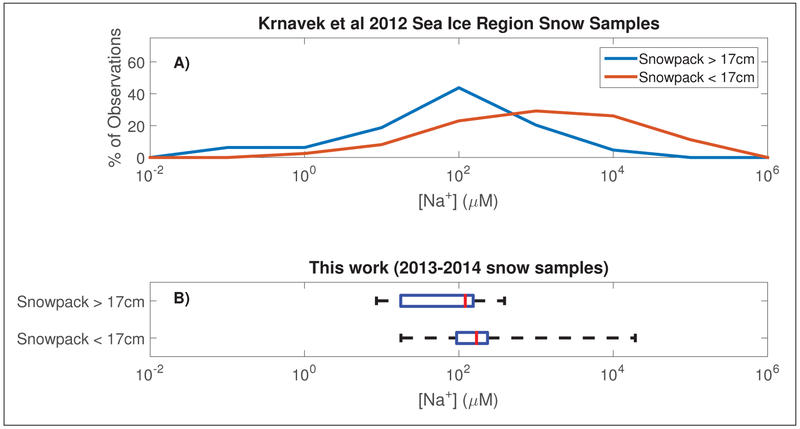
The salinity of the surface snow observed in this study, as described by sodium concentrations (Na+) (Figure 3), is consistent with prior findings that brine on sea ice can only migrate a limited distance (<17 cm) into the overlying snowpack (Domine et al., 2004). Samples from both the current work and the prior Krnavek et al. (2012) study show that surface snow collected from snowpacks less than 17 cm deep had higher sodium concentrations than those collected from deeper snowpacks (Figure 3). This difference in salinity is significant at the 95% confidence level (p = 4.2∙10−10) for the larger set of Krnavek et al. (2012) samples. Snowpack vertical profiles collected at five sites (Figure S1) also show this trend of decreasing salinity with increased distance from the sea ice surface. The low sodium content of the surface samples collected from deeper snowpacks in this work suggests that the presence of halides in these samples is due to the deposition of SSA from the atmosphere and/or deposition of halogen-containing gas phase compounds, rather than wicking of brine from the sea ice surface.
Figure 3:
Distributions of snow salinity as represented by sodium concentrations are shown for A) the surface snow samples collected by Krnavek et al. (2012) and B) the 29 samples collected in this work. In panel B, the red line represents the median, the blue box is the inner two quartiles, and whiskers span the outer two quartiles. The cut-point of 17 cm was chosen based on the upper limit depth at which brine wicking minimally influences surface snow salinity (Domine et al., 2004). DOI: https://doi.org/10.1525/elementa.352.f3
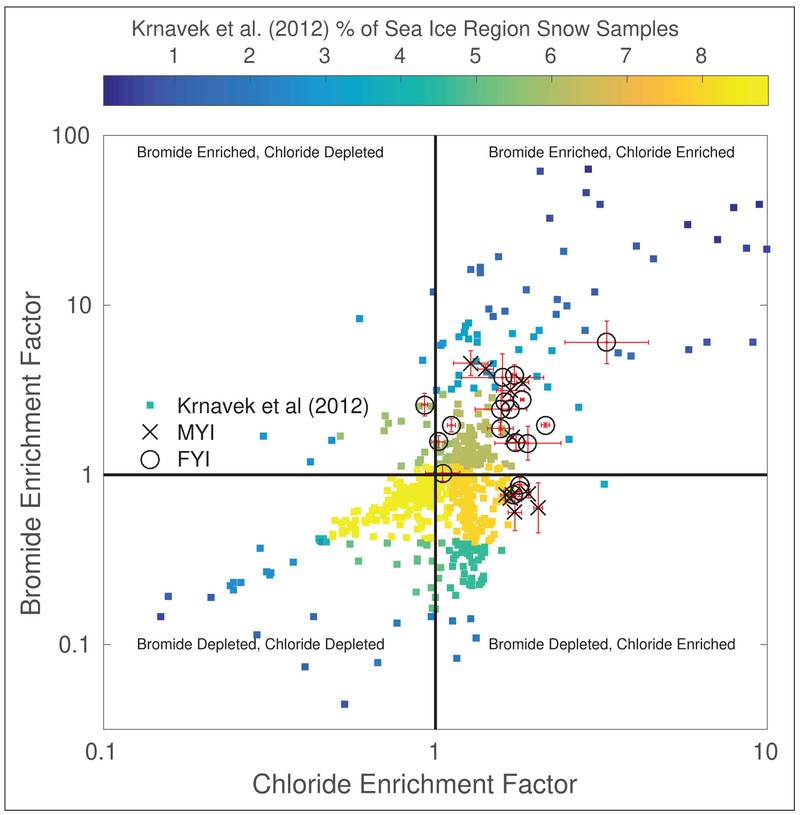
To determine relative deviations from standard seawater ratios, we calculated halide (X) enrichment factors (Ef) for bromide and chloride relative to sodium (Ef[X]) (Figure 4), according to Newberg et al. (2005).
| (1) |
where [X] corresponds to the concentration of bromide or chloride and [Na+] is the concentration of sodium, in the snow samples and seawater, respectively. Enrichment factors greater than one indicate enrichment relative to seawater, while values lower than one indicate depletion relative to seawater. Snow from both MYI and FYI regions displayed comparable average levels of chloride enrichment (median of 1.7 for both regions). From these 29 samples, there is no relationship between the sea ice environment where the sample was collected and the level of chloride enrichment. For the 29 samples in this study, surface snow samples from MYI and FYI regions displayed differing levels of bromide enrichment with median enrichment factors of 0.8 and 2.0, respectively (Figure 4). In total, 20 of 29 surface snow samples in both FYI and MYI regions were enriched in bromide (Figure 4), indicating bromide enrichment is more common than bromide depletion. Although in this study, bromide depletion was more commonly observed in MYI regions (6 of 11 samples), indicating it had served as a source of atmospheric bromine. Snow in FYI regions was more often enriched (15 of 18 samples), reflecting the deposition of bromine-containing trace gases. These results suggest the salinity of the underlying ice is not as important as atmospheric processes in determining surface snowpack halide concentrations in central Arctic sea ice regions. These findings are consistent with prior studies in the Arctic showing increasing bromide enrichment in snowpacks 300 km inland, indicating that bromide from sea ice regions had been transported and deposited further inland (Simpson et al., 2005). As with the prior measurements by Krnavek et al. (2012), these data also show that bromide concentrations at low salinities are dominated by atmospheric exchange.
Figure 4:
Enrichment factors relative to seawater for bromide and chloride for each snow sample, grouped by the ice region where the sample was collected, and also showing snow samples collected in sea ice regions north of Alaska (Krnavek et al., 2012). Enrichment factors greater than one indicate enrichment relative to seawater, while values lower than one indicate depletion relative to seawater. The scatter plot is divided into four regimes based on enrichment of the two ions relative to seawater. Uncertainties in enrichment factors, calculated from standard deviations of triplicate IC measurements of a single sample, are plotted in red. The Krnavek et al. (2012) data are colored by the percentage of observations, with more commonly observed enrichment factors plotted in yellow and less common enrichment factors plotted in blue. DOI: https://doi.org/10.1525/elementa.352.f4
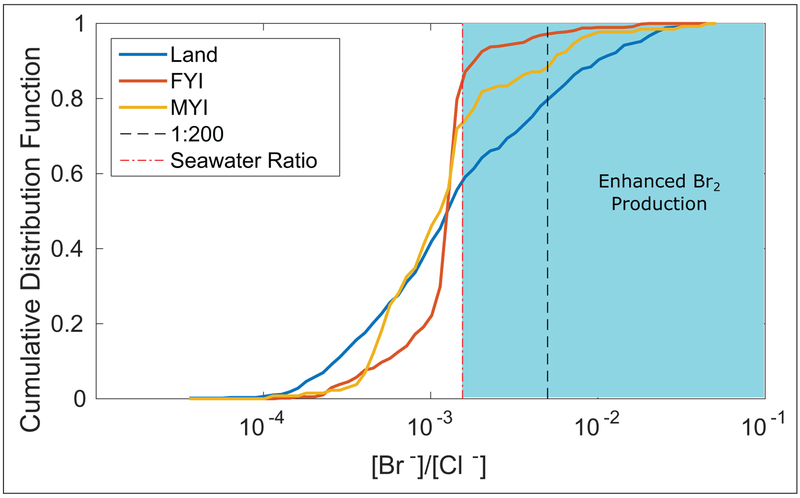
The observed deviations from seawater ratios are reflective of the widespread atmospheric bromine chemistry occurring in the Arctic boundary layer. Snowpacks serve as both a sink and source of atmospheric bromine by providing a surface for trace gas deposition and serving as a source of Br2 and BrCl through multiphase reactions (Custard et al., 2017). It is likely the net enrichment of bromide observed at the time of sampling reflects both processes occurring; thus, we interpret observations of both depletion and enhancement of bromide in both FYI and MYI regions as evidence that snowpacks in both regions are actively serving as both sources and sinks of reactive bromine to the atmosphere. Pratt et al. (2013) and Custard et al. (2017) showed that the ratio of Br−/Cl− in the snowpack is important in determining snowpack Br2, BrCl, and Cl2 production. Figure 5 displays the cumulative distribution function for surface snow Br−/Cl− derived from the Krnavek et al. (2012) dataset for surface snow over land, FYI, and MYI. Snowpacks in both terrestrial and MYI regions exhibit ratios favorable to Br2 production (>1:200, Pratt et al. (2013)) more often than FYI regions (25% and 15% vs 5%, respectively). This trend is also observed when considering any amount of bromide enrichment, with 40% of terrestrial snowpacks and 26% of MYI snowpacks being enriched in bromide, vs. only 14% of FYI snowpacks. This finding underscores the role of atmospheric processing, both deposition and production of bromine-containing gases, in modifying the chemical composition of these snowpacks, as opposed to those in FYI regions which tend to be more saline and are not as likely to exhibit enrichments or depletions in bromide compared to seawater.
Figure 5:
Cumulative distribution functions calculated from the Krnavek et al. (2012) dataset for Br−/Cl− mole ratios in snow collected over land (blue), FYI regions (red), and MYI regions (yellow). The dashed red line denotes the sea water ratio (Millero et al., 2008) of these anions, and the black line denotes a ratio of 1:200, which has been observed as optimal for Br2 production (Pratt et al., 2013), in agreement with laboratory studies by (Huff and Abbatt, 2002; Adams et al., 2002; Sjostedt and Abbatt, 2008) showing bromide enrichment is required for Br2 production. DOI: https://doi.org/10.1525/elementa.352.f5
Similarly, 28 of the 29 surface snow samples collected in this study were enriched in chloride, suggesting deposition of chlorine-containing trace gases enhances snowpack chloride concentrations in both FYI and MYI regions. Chloride enriched snowpacks serve as a source of both Cl2 and BrCl to the atmosphere, but the production of these Cl2 is reduced below the eutectic point (−22.9°C) of hydrohalite (NaCl·2H2O), where hydrohalite precipitation limits chloride availability for chlorine activation (Sjostedt and Abbatt, 2008; Custard et al., 2017). The majority of samples were collected during February and April, when air temperatures are typically below this eutectic temperature (Rigor et al., 2000), likely leading to a lack of observed chloride depletion.
Recommendations for the modeling community
Determining the role of FYI and MYI regions and future impacts of ongoing Arctic cryospheric changes on snowpack driven halogen chemistry over a pan-Arctic scale is suited to a modeling approach (e.g. Yang et al., 2010; Toyota et al., 2011; Falk and Sinnhuber, 2018). However, the lack of halide measurements in remote Arctic sea ice locations prior to this study have required disparate assumptions to be made about snowpack salinity and the ability of various sea ice regions to initiate and sustain halogen activation. For example, Yang et al. (2010) use Antarctic composition data to approximate Arctic snow salinity and assumed no production of reactive bromine from MYI regions due to the lower salinity of the snowpack. Toyota et al. (2011) included bromine release from both FYI and MYI regions, finding that modeling bromine release happening more efficiently in FYI regions than MYI regions produced improved model agreement with satellite-based BrO observations. Falk and Sinnhuber (2018) extended the Toyota et al. (2011) paramaterization to both the Arctic and Antractic over a full year, rather than just the polar spring. They found good agreement with GOME-2 BrO tropospheric vertical column density (VCD) observations, although the model generally under-estimates the observed VCDs. The bromide measurements presented in this work suggest that the surface snowpack in both FYI and MYI regions can serve as a source of Br2, with the majority of samples being enriched in bromide (Figure 4), a prerequisite for bromine activation (Pratt et al., 2013), presumably due the deposition of trace bromine gases (Simpson et al., 2005). This finding suggests the approach of Toyota et al. (2011) and Falk and Sinnhuber (2018), with Br2 production from both FYI and MYI surface snow, is more appropriate than only considering activation from FYI regions.
Current modeling work only considers snowpack bulk salinity as a relevant parameter, but recent laboratory and field measurements suggest that other properties such as pH (Wren et al., 2013; Pratt et al., 2013) and Br−/Cl− ratio (Pratt et al., 2013) are more important than bulk salinity for Br2 production. While pH measurements across sea ice regions remain limited, the pH of snow measured in this work ranged from 4.8–6.6, corresponding to acidic snowpacks which would support halogen activation (Wren et al., 2013; Pratt et al., 2013). The relationship of bulk snow melt water pH and ion concentrations to the values at the snow grain surface should also be explored, as the halides may not be present at the surface (Bartels-Rausch et al., 2012), or may not uniformly cover the snow grain surface (Malley et al., 2018). The Br−/Cl− ratios presented in Figure 5 suggest the ability of terrestrial and MYI snowpacks to produce Br2 should be accounted for in models. Airborne measurements of BrO 200 km inland by Peterson et al. (2018) provide further evidence of the effectiveness of terrestrial snowpacks in producing reactive bromine. Given that the conditions for Br2 production are most frequently observed in terrestrial snowpacks, it is likely those snowpacks will play an increasing role in Arctic boundary layer bromine chemistry as sea ice extent declines.
Conclusions
We measured concentrations of chloride, bromide, and sodium in snow meltwater samples from FYI and MYI regions near Greenland, Alaska, and in the central Arctic. We find surface snow from both MYI regions and FYI regions is generally enriched in chloride compared to seawater. Snow in FYI regions is most often enriched in bromide, while snow in MYI regions shows both depletion and enrichment of bromide. These findings suggest that snow in both MYI and FYI regions is playing a role in bromine and chlorine activation chemistry and its associated impacts. The snow meltwater bromide concentrations and deviation from seawater ratios observed in this study point to snowpacks in MYI ice regions being a source of Br2. This finding is supported by measurements of ozone and BrO in predominantly MYI regions showing both boundary layer ozone depletion events (e.g. Gilman et al., 2010; Halfacre et al., 2014) and increased amounts of atmospheric BrO compared to FYI and coastal regions (Peterson et al., 2016), pointing to a role for MYI regions in initiating and sustaining active bromine chemistry. Recent observations by Peterson et al. (2017) of a lofted bromine plume over Utqiaġvik, Alaska also implicated the snowpack in MYI regions as the initial source of reactive bromine. In recent years, the fraction of first-year sea ice (FYI) cover in the Arctic Ocean has increased, while multi-year sea ice (MYI) extent has decreased (Nghiem et al., 2007; Maslanik et al., 2011). Reductions in overall sea ice extent are also expected to lead to increased snowfall in coastal Arctic regions (Bhatt et al., 2014). These changes in sea ice coverage impact the overlying snowpack (Webster et al., 2014), influencing boundary layer bromine chemistry and likely chemistry occurring aloft due to atmospheric mixing (Peterson et al., 2017).
Given the relatively low tropospheric hydroxyl radical levels in the Arctic basin (Saiz-Lopez and von Glasow, 2012), halogen radicals play an important role in the oxidation of atmospheric pollutants in the springtime polar boundary layer (Simpson et al., 2007b). Despite the importance of chlorine radicals in the Arctic boundary layer, measurements of atmospheric trace chlorine gases remain limited (e.g. Impey et al., 1997, 1999; Keil and Shepson, 2006; Liao et al., 2014; Custard et al., 2016). Our finding of widespread chloride enrichment suggests that snowpack driven chlorine production at temperatures above the hydrohalite eutectic temperature (−22.9°C) (Custard et al., 2017) would likely occur in both MYI and FYI regions, playing a key role in the oxidation of volatile organic compounds (Jobson et al., 1994; Keil and Shepson 2006), including methane, a greenhouse gas, over the entire sea ice covered Arctic. This study underscores the need for measurements of chlorine-containing trace gases, as well as the chloride content of aerosols and the snowpack, in remote sea ice regions.
Measurements of snowpack salinity and snowpack depth suggest that trace gas deposition, rather than the wicking of brine from the sea ice surface, is the primary source of excess halides in the surface snowpack in both FYI and MYI regions. This finding provides an explanation for observations of enhanced tropospheric BrO in multi-year ice regions (Peterson et al., 2016; Burd et al., 2017). Our measurements suggest the modeling of atmospheric halogen chemistry on a pan-Arctic scale should take into account surface snowpack halogen production from FYI regions, as well as MYI and snow-covered coastal regions (e.g. Falk and Sinnhuber, 2018). Given the rapid decline of sea ice extent, particularly the loss of MYI regions, ascertaining the role of these MYI and inland snow-covered regions as compared with FYI regions in boundary layer halogen chemistry is a critical need, which requires more halogen measurements in MYI regions.
Supplementary Material
• Figure S1. Profiles of bromide and chloride within the snowpack at 5 sites. The seawater ratio (Millero et al., 2008) is plotted for reference. DOI: https://doi.org/10.1525/elementa.352.s1
• Table S1. Sample pH, location, snowpack depth, sampling depth, and sea ice type. DOI: https://doi.org/10.1525/elementa.352.s1
• Table S2. Sample ion concentrations (μM) and uncertainties for Na+ Mg2+, Ca2+, K+, Cl−, SO42−, Br−, and NO3−. DOI: https://doi.org/10.1525/elementa.352.s1
Acknowledgements
Michael Weber, Natalie Cleveland, and Eric Boone (University of Michigan) are thanked for assistance with IC analysis. Melinda Webster (University of Washington) is thanked for assistance shipping samples to University of Michigan. Bill Simpson and Laura Krnavek (University of Alaska Fairbanks) are thanked for the provision of additional snowpack composition data (Krnavek et al., 2012).
Funding information
Financial and logistical support for sample collection was provided by the National Science Foundation (NSF)’s North Pole Environmental Observatory (ARC-0856330) and Switchyard program (ARC-1022475). The work at University of Michigan was supported by the National Aeronautics and Space Administration (NASA) Atmospheric Composition Program (NNX14AP44G), Michigan Space Grant Consortium, and the University of Michigan Department of Chemistry. Rigor was funded by NSF, Shell Inc., and other contributors to the US Interagency Arctic Buoy Program. The research at the Jet Propulsion Laboratory, California Institute of Technology, was supported by the NASA Cryospheric Sciences Program and the Atmospheric Composition Program.
Footnotes
Data Accessibility Statement
Prior snowpack composition data, first published in Krnavek et al. (2012), are archived at the NSF Arctic Data Center (https://doi.org/10.5065/D6930R9W) (Sturm et al. 2016). Snowpack composition data first presented in this manuscript are also archived at the NSF Arctic Data Center (https://doi.org/10.18739/A2G56H) (Peterson and Pratt 2017).
Supplemental files
The supplemental files for this article can be found as follows:
Competing interests
The authors have no competing interests to declare.
References
- Abbatt JPD, Thomas JL, Abrahamsson K, Boxe C, Granfors A, Jones AE, King MD, Saiz-Lopez A, Shepson PB, Sodeau J, Toohey DW, Toubin C, von Glasow R, Wren SN and Yang X 2012. Halogen activation via interactions with environmental ice and snow in the polar lower troposphere and other regions. Atmospheric Chemistry and Physics 12(14): 6237–6271. ISSN 1680–7324 http://www.atmos-chem-phys.net/12/6237/2012/. DOI: 10.5194/acp-12-6237-2012 [DOI] [Google Scholar]
- Adams JW, Holmes NS and Crowley JN. 2002. Uptake and reaction of HOBr on frozen and dry NaCl/NaBr surfaces between 253 and 233 K. Atmospheric Chemistry and Physics 2(1): 79–91. ISSN 1680–7324 http://www.atmos-chem-phys.net/2/79/2002/. DOI: 10.5194/acp-2-79-2002 [DOI] [Google Scholar]
- Barrie LA, Bottenheim JW, Schnell RC, Crutzen PJ and Rasmussen RA. 1988. Ozone destruction and photochemical reactions at polar sunrise in the lower Arctic atmosphere. Nature 334(6178): 138–141. ISSN 0028–0836 http://www.nature.com/doifinder/10.1038/334138a0. DOI: 10.1038/334138a0 [DOI] [Google Scholar]
- Bartels-Rausch T, Bergeron V, Cartwright JHE, Escribano R, Finney JL, Grothe H, Gutiérrez PJ, Haapala J, Kuhs WF, Pettersson JBC, Price SD, Sainz-Díaz CI, Stokes DJ, Strazzulla G, Thomson ES, Trinks H and Uras-Aytemiz N 2012. Ice structures, patterns, and processes: A view across the icefields. Reviews of Modern Physics 84(2): 885–944. ISSN 0034–6861 http://journals.aps.org/rmp/abstract/10.1103/RevModPhys.84.885. DOI: 10.1103/RevModPhys.84.885 [DOI] [Google Scholar]
- Bhatt US, Walker DA, Walsh JE, Carmack EC, Frey KE, Meier WN, Moore SE, Parmentier FJW, Post E, Romanovsky VE and Simpson WR. 2014. Implications of Arctic Sea Ice Decline for the Earth System. Annual Review of Environment and Resources 39(1): 57–89. ISSN 1543–5938 http://www.annualreviews.org/doi/abs/10.1146/annurev-environ-122012-094357. DOI: 10.1146/annurev-environ-122012-094357 [DOI] [Google Scholar]
- Blanchard-Wrigglesworth E, Farrell SL, Newman T and Bitz CM. 2015. Snow cover on Arctic sea ice in observations and an Earth System Model. Geophysical Research Letters 42(23): 10,342–10,348. ISSN 00948276 http://doi.wiley.com/10.1002/2015GL066049. DOI: 10.1002/2015GL066049 [DOI] [Google Scholar]
- Browse J, Carslaw KS, Mann GW, Birch CE, Arnold SR and Leck C 2014. The complex response of Arctic aerosol to sea-ice retreat. Atmospheric Chemistry and Physics 14(14): 7543–7557. ISSN 1680–7324 http://www.atmos-chem-phys.net/14/7543/2014/acp-14-7543-2014.html. DOI: 10.5194/acp-14-7543-2014 [DOI] [Google Scholar]
- Burd JA, Peterson PK, Nghiem SV, Perovich DK and Simpson WR. 2017. Snowmelt onset hinders bromine monoxide heterogeneous recycling in the Arctic. Journal of Geophysical Research: Atmospheres 122(15): 8297–8309. ISSN 2169897X http://doi.wiley.com/10.1002/2017JD026906. DOI: 10.1002/2017JD026906 [DOI] [Google Scholar]
- Cox GF and Weeks W 1973. Salinity variations in sea ice. Journal of Glaciology 13(67): 109–120. http://oai.dtic.mil/oai/oai?verb=getRecord&metadataPrefix=html&identifier=AD0768170. DOI: 10.1017/S0022143000023418 [DOI] [Google Scholar]
- Custard KD, Pratt KA, Wang S and Shepson PB. 2016. Constraints on Arctic Atmospheric Chlorine Production through Measurements and Simulations of Cl2 and ClO. Environmental Science & Technology 50(22): 12394–12400. ISSN 0013–936X http://pubs.acs.org/doi/10.1021/acs.est.6b03909. DOI: 10.1021/acs.est.6b03909 [DOI] [PubMed] [Google Scholar]
- Custard KD, Raso ARW, Shepson PB, Staebler RM and Pratt KA. 2017. Production and Release of Molecular Bromine and Chlorine from the Arctic Coastal Snowpack. ACS Earth and Space Chemistry 1(3): 142–151. ISSN 2472–3452 http://pubs.acs.org/doi/abs/10.1021/acsearthspacechem.7b00014. DOI: 10.1021/acsearthspacechem.7b00014 [DOI] [Google Scholar]
- de Caritat P, Hall G, Gìslason S, Belsey W, Braun M, Goloubeva NI, Olsen HK, Scheie JO and Vaive JE. 2005. Chemical composition of arctic snow: Concentration levels and regional distribution of major elements. Science of the Total Environment 336(1–3): 183–199. ISSN 00489697 http://www.sciencedirect.com/science/article/pii/S004896970400467X. DOI: 10.1016/j.scitotenv.2004.05.031 [DOI] [PubMed] [Google Scholar]
- DeMott PJ, Hill TCJ, McCluskey CS, Prather KA, Collins DB, Sullivan RC, Ruppel MJ, Mason RH, Irish VE, Lee T, Hwang CY, Rhee TS, Snider JR, McMeeking GR, Dhaniyala S, Lewis ER, Wentzell JJB, Abbatt J, Lee C, Sultana CM, Ault AP, Axson JL, Diaz, Martinez M, Venero I, Santos-Figueroa G, Stokes MD, Deane GB, Mayol-Bracero OL, Grassian VH, Bertram TH, Bertram AK, Moffett BF and Franc GD. 2016. Sea spray aerosol as a unique source of ice nucleating particles. Proceedings of the National Academy of Sciences 113(21): 5797–5803. ISSN 0027–8424 http://www.pnas.org/lookup/doi/10.1073/pnas.1514034112. DOI: 10.1073/pnas.1514034112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibb JE, Ziemba LD, Luxford J and Beckman P 2010. Bromide and other ions in the snow, firn air, and atmospheric boundary layer at Summit during GSHOX. Atmospheric Chemistry and Physics 10(20): 9931–9942. ISSN 1680–7324 http://www.atmos-chem-phys.net/10/9931/2010/acp-10-9931-2010.html. DOI: 10.5194/acp-10-9931-2010 [DOI] [Google Scholar]
- Domine F and Shepson PB. 2002. Air-Snow Interactions and Atmospheric Chemistry. Science 297(5586): 1506–1510. ISSN 00368075 http://www.sciencemag.org/cgi/doi/10.1126/science.1074610. DOI: 10.1126/science.1074610 [DOI] [PubMed] [Google Scholar]
- Domine F, Sparapani R, Ianniello A and Beine HJ. 2004. The origin of sea salt in snow on Arctic sea ice and in coastal regions. Atmospheric Chemistry and Physics 4(9/10): 2259–2271. ISSN 1680–7324 http://www.atmos-chem-phys.net/4/2259/2004/. DOI: 10.5194/acp-4-2259-2004 [DOI] [Google Scholar]
- Douglas TA and Sturm M 2004. Arctic haze, mercury and the chemical composition of snow across northwestern Alaska. Atmospheric Environment 38(6): 805–820. ISSN 13522310 http://www.sciencedirect.com/science/article/pii/S135223100300949X. DOI: 10.1016/j.atmosenv.2003.10.042 [DOI] [Google Scholar]
- Falk S and Sinnhuber BM. 2018. Polar boundary layer bromine explosion and ozone depletion events in the chemistry–climate model EMAC v2.52: implementation and evaluation of AirSnow algorithm. Geoscientific Model Development 11(3): 1115–1131. ISSN 1991–9603 https://www.geosci-model-dev.net/11/1115/2018/. DOI: 10.5194/gmd-11-1115-2018 [DOI] [Google Scholar]
- Gilman JB, Burkhart JF, Lerner BM, Williams EJ, Kuster WC, Goldan PD, Murphy PC, Warneke C, Fowler C, Montzka SA, Miller BR, Miller L, Oltmans SJ, Ryerson TB, Cooper OR, Stohl A and de Gouw JA. 2010. Ozone variability and halogen oxidation within the Arctic and sub-Arctic springtime boundary layer. Atmospheric Chemistry and Physics 10(21): 10223–10236. ISSN 1680–7324 http://www.atmos-chem-phys.org/10/10223/2010/acp-10-10223-2010.html. DOI: 10.5194/acp-10-10223-2010 [DOI] [Google Scholar]
- Halfacre JW, Knepp TN, Shepson PB, Thompson CR, Pratt KA, Li B, Peterson PK, Walsh SJ, Simpson WR, Matrai PA, Bottenheim JW, Netcheva S, Perovich DK and Richter A 2014. Temporal and spatial characteristics of ozone depletion events from measurements in the Arctic. Atmospheric Chemistry and Physics 14(10): 4875–4894. ISSN 1680–7324 http://www.atmos-chem-phys.net/14/4875/2014/acp-14-4875-2014.html. DOI: 10.5194/acp-14-4875-2014 [DOI] [Google Scholar]
- Harris DC. 2016. Quantitative Chemical Analysis. 9th ed New York: W. H. Freeman and Company; ISBN 978-1-4641-3538-5. [Google Scholar]
- Huff AK and Abbatt JPD. 2002. Kinetics and Product Yields in the Heterogeneous Reactions of HOBr with Ice Surfaces Containing NaBr and NaCl. The Journal of Physical Chemistry A 106(21): 5279–5287. ISSN 1089–5639 http://pubs.acs.org/doi/abs/10.1021/jp014296m. DOI: 10.1021/jp014296m [DOI] [Google Scholar]
- Impey G, Mihele C, Anlauf K, Barrie L, Hastie D and Shepson P 1999. Measurements of Photolyzable Halogen Compounds and Bromine Radicals During the Polar Sunrise Experiment 1997. Journal of Atmospheric Chemistry 34(1): 21–37. ISSN 01677764 http://link.springer.com/10.1023/A:1006264912394. DOI: 10.1023/A:1006264912394 [DOI] [Google Scholar]
- Impey GA, Shepson PB, Hastie DR, Barrie LA and Anlauf KG. 1997. Measurements of photolyzable chlorine and bromine during the Polar Sunrise Experiment 1995. DOI: 10.1029/97JD00851 [DOI] [Google Scholar]
- Jacobi HW, Voisin D, Jaffrezo JL, Cozic J and Douglas TA. 2012. Chemical composition of the snowpack during the OASIS spring campaign 2009 at Barrow, Alaska. Journal of Geophysical Research 117: D00R13 ISSN 0148–0227 http://doi.wiley.com/10.1029/2011JD016654. DOI: 10.1029/2011JD016654 [DOI] [Google Scholar]
- Jobson BT, Niki H, Yokouchi Y, Bottenheim J, Hopper F and Leaitch R 1994. Measurements of C2–C6 hydrocarbons during the Polar Sunrise1992 Experiment: Evidence for Cl atom and Br atom chemistry. Journal of Geophysical Research 99(D12): 25355 ISSN 0148–0227 http://doi.wiley.com/10.1029/94JD01243. DOI: 10.1029/94JD01243 [DOI] [Google Scholar]
- Keil AD and Shepson PB. 2006. Chlorine and bromine atom ratios in the springtime Arctic troposphere as determined from measurements of halogenated volatile organic compounds. Journal of Geophysical Research Atmospheres 111(17): 1–11. ISSN 01480227 DOI: 10.1029/2006JD00711920411040 [DOI] [Google Scholar]
- Koo JH, Wang Y, Kurosu TP, Chance K, Rozanov A, Richter A, Oltmans SJ, Thompson AM, Hair JW, Fenn MA, Weinheimer AJ, Ryerson TB, Solberg S, Huey LG, Liao J, Dibb JE, Neuman JA, Nowak JB, Pierce RB, Natarajan M and Al-Saadi J 2012. Characteristics of tropospheric ozone depletion events in the Arctic spring: analysis of the ARCTAS, ARCPAC, and ARCIONS measurements and satellite BrO observations. Atmospheric Chemistry and Physics 12(20): 9909–9922. ISSN 1680–7324 http://www.atmos-chem-phys.net/12/9909/2012/acp-12-9909-2012.html. DOI: 10.5194/acp-12-9909-2012 [DOI] [Google Scholar]
- Krnavek L, Simpson WR, Carlson D, Domine F, Douglas TA and Sturm M 2012. The chemical composition of surface snow in the Arctic: Examining marine, terrestrial, and atmospheric influences. Atmospheric Environment 50(0): 349–359. ISSN 13522310 http://www.sciencedirect.com/science/article/pii/S1352231011012192. DOI: 10.1016/j.atmosenv.2011.11.033 [DOI] [Google Scholar]
- Leck C, Norman M, Bigg EK and Hillamo R 2002. Chemical composition and sources of the high Arctic aerosol relevant for cloud formation. Journal of Geophysical Research 107(D12): 4135 ISSN 0148–0227 http://doi.wiley.com/10.1029/2001JD001463. DOI: 10.1029/2001JD001463 [DOI] [Google Scholar]
- Lewis ER and Schwartz SE. 2004. Sea Salt Aerosol Production: Mechanisms, Methods, Measurements, and Models – A Critical Review. American Geophysical Union. ISBN 978–0875904177. [Google Scholar]
- Liao J, Huey LG, Liu Z, Tanner DJ, Cantrell CA, Orlando JJ, Flocke FM, Shepson PB, Weinheimer AJ, Hall SR, Ullmann K, Beine HJ, Wang Y, Ingall ED, Stephens CR, Hornbrook RS, Apel EC, Riemer D, Fried A, Mauldin III RL, Smith JN, Staebler RM, Neuman JA and Nowak JB. 2014. High levels of molecular chlorine in the Arctic atmosphere. Nature Geosci 7(2): 91–94. DOI: 10.1038/ngeo2046 [DOI] [Google Scholar]
- Macdonald KM, Sharma S, Toom D, Chivulescu A, Hanna S, Bertram AK, Platt A, Elsasser M, Huang L, Tarasick D, Chellman N, McConnell JR, Bozem H, Kunkel D, Lei YD, Evans GJ and Abbatt JPD. 2017. Observations of atmospheric chemical deposition to high Arctic snow. Atmospheric Chemistry and Physics 17(9): 5775–5788. ISSN 1680–7324 http://www.atmos-chem-phys.net/17/5775/2017/. DOI: 10.5194/acp-17-5775-2017 [DOI] [Google Scholar]
- Malley PPA, Chakraborty S and Kahan TF. 2018. Physical Characterization of Frozen Saltwater Solutions using Raman Microscopy. ACS Earth and Space Chemistry. ISSN 2472–3452 http://pubs.acs.org/doi/10.1021/acsearthspacechem.8b00045. DOI: 10.1021/acsearthspacechem.8b00045 [DOI] [Google Scholar]
- Maslanik J, Stroeve J, Fowler C and Emery W 2011. Distribution and trends in Arctic sea ice age through spring 2011. Geophysical Research Letters 38(13): L13502 ISSN 00948276 http://doi.wiley.com/10.1029/2011GL047735. DOI: 10.1029/2011GL047735 [DOI] [Google Scholar]
- May NW, Quinn PK, McNamara SM and Pratt KA. 2016. Multiyear study of the dependence of sea salt aerosol on wind speed and sea ice conditions in the coastal Arctic. Journal of Geophysical Research: Atmospheres 121(15): 9208–9219. ISSN 2169897X http://doi.wiley.com/10.1002/2016JD025273. DOI: 10.1002/2016JD025273 [DOI] [Google Scholar]
- Millero FJ, Feistel R, Wright DG and McDougall TJ. 2008. The composition of Standard Seawater and the definition of the Reference-Composition Salinity Scale. Deep-Sea Research Part I: Oceanographic Research Papers 55(1): 50–72. ISSN 09670637 DOI: 10.1016/j.dsr.2007.10.001 [DOI] [Google Scholar]
- Newberg JT, Matthew BM and Anastasio C 2005. Chloride and bromide depletions in sea-salt particles over the northeastern Pacific Ocean. Journal of Geophysical Research: Atmospheres 110(D6). ISSN 01480227 http://doi.wiley.com/10.1029/2004JD005446. DOI: 10.1029/2004JD005446 [DOI] [Google Scholar]
- Nghiem SV, Chao Y, Neumann G, Li P, Perovich DK, Street T and Clemente-Colón P 2006. Depletion of perennial sea ice in the East Arctic Ocean. Geophysical Research Letters 33(17): L17501 ISSN 0094–8276 http://doi.wiley.com/10.1029/2006GL027198. DOI: 10.1029/2006GL027198 [DOI] [Google Scholar]
- Nghiem SV, Rigor IG, Perovich DK, Clemente-Colón P, Weatherly JW and Neumann G 2007. Rapid reduction of Arctic perennial sea ice. Geophysical Research Letters 34(19): L19504 ISSN 0094–8276 http://doi.wiley.com/10.1029/2007GL031138. DOI: 10.1029/2007GL031138 [DOI] [Google Scholar]
- Nghiem SV, Van Woert ML and Neumann G 2005. Rapid formation of a sea ice barrier east of Svalbard. Journal of Geophysical Research 110(C11): C11013 ISSN 0148–0227 http://doi.wiley.com/10.1029/2004JC002654. DOI: 10.1029/2004JC002654 [DOI] [Google Scholar]
- Nilsson ED and Rannik Ü 2001. Turbulent aerosol fluxes over the Arctic Ocean: 1. Dry deposition over sea and pack ice. Journal of Geophysical Research 106(D23): 32125 ISSN 0148–0227 http://doi.wiley.com/10.1029/2000JD900605. DOI: 10.1029/2000JD900605 [DOI] [Google Scholar]
- Nilsson ED, Rannik Ü, Swietlicki E, Leck C, Aalto PP, Zhou J and Norman M 2001. Turbulent aerosol fluxes over the Arctic Ocean: 2. Wind-driven sources from the sea. Journal of Geophysical Research 106(D23): 32139 ISSN 0148–0227 http://doi.wiley.com/10.1029/2000JD900747. DOI: 10.1029/2000JD900747 [DOI] [Google Scholar]
- Perovich DK and Richter-Menge JA. 1994. Surface characteristics of lead ice. Journal of Geophysical Research 99(C8): 16341 ISSN 0148–0227 http://doi.wiley.com/10.1029/94JC01194. DOI: 10.1029/94JC01194 [DOI] [Google Scholar]
- Peterson PK, Pöhler D, Sihler H, Zielcke J, General S, Frieß U, Platt U, Simpson WR, Nghiem SV, Shepson PB, Stirm BH, Dhaniyala S, Wagner T, Caulton DR, Fuentes JD and Pratt KA. 2017. Observations of bromine monoxide transport in the Arctic sustained on aerosol particles. Atmospheric Chemistry and Physics 17(12): 7567–7579. ISSN 1680–7324 http://www.atmos-chem-phys.net/17/7567/2017/. DOI: 10.5194/acp-17-7567-2017 [DOI] [Google Scholar]
- Peterson PK, Pöhler D, Zielcke J, General S, Frieß U, Platt U, Simpson WR, Nghiem SV, Shepson PB, Stirm BH and Pratt KA. 2018. Springtime Bromine Activation over Coastal and Inland Arctic Snowpacks. ACS Earth and Space Chemistry 2(10): 1075–1086. ISSN 2472–3452 http://pubs.acs.org/doi/10.1021/acsearthspacechem.8b00083. DOI: 10.1021/acsearthspacechem.8b00083 [DOI] [Google Scholar]
- Peterson PK and Pratt KA. 2017. Central Arctic snow chemical composition, 2013–2014. DOI: 10.18739/A2G56H [DOI] [Google Scholar]
- Peterson PK, Simpson WR and Nghiem SV. 2016. Variability of bromine monoxide at Barrow, Alaska, over four halogen activation (March–May) seasons and at two on-ice locations. Journal of Geophysical Research: Atmospheres 121(3): 1381–1396. ISSN 2169897X http://onlinelibrary.wiley.com/doi/10.1002/2015JD024094/abstract. DOI: 10.1002/2015JD024094 [DOI] [Google Scholar]
- Piot M and von Glasow R 2008. The potential importance of frost flowers, recycling on snow, and open leads for ozone depletion events. Atmospheric Chemistry and Physics 8(9): 2437–2467. ISSN 1680–7324 http://www.atmos-chem-phys.net/8/2437/2008/acp-8-2437-2008.html. DOI: 10.5194/acp-8-2437-2008 [DOI] [Google Scholar]
- Platt U, Allan W and Lowe D 2004. Hemispheric average Cl atom concentration from 13C/12C ratios in atmospheric methane. Atmospheric Chemistry and Physics 4(9/10): 2393–2399. ISSN 1680–7324 http://www.atmos-chem-phys.net/4/2393/2004/. DOI: 10.5194/acp-4-2393-2004 [DOI] [Google Scholar]
- Pratt KA, Custard KD, Shepson PB, Douglas TA, Pöhler D, General S, Zielcke J, Simpson WR, Platt U, Tanner DJ, Gregory, Huey L, Carlsen M and Stirm BH. 2013. Photochemical production of molecular bromine in Arctic surface snowpacks. Nature Geoscience 6(5): 351–356. ISSN 1752–0894 http://www.nature.com/doifinder/10.1038/ngeo1779. DOI: 10.1038/ngeo1779 [DOI] [Google Scholar]
- Raso ARW, Custard KD, May NW, Tanner D, Newburn MK, Walker L, Moore RJ, Huey LG, Alexander L, Shepson PB and Pratt KA. 2017. Active molecular iodine photochemistry in the Arctic. Proceedings of the National Academy of Sciences of the United States of America. ISSN 1091–6490 http://www.ncbi.nlm.nih.gov/pubmed/28874585. DOI: 10.1073/pnas.1702803114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigor IG, Colony RL and Martin S 2000. Variations in surface air temperature observations in the Arctic. Journal of Climate 13(5): 896–914. ISSN 0894–8755 http://journals.ametsoc.org/doi/abs/10.1175/1520-0442%282000%29013%3C0896%3AVISATO%3E2.0.CO%3B2. DOI: [DOI] [Google Scholar]
- Saiz-Lopez A and von Glasow R 2012. Reactive halogen chemistry in the troposphere. Chemical Society Reviews 41(19): 6448 ISSN 0306–0012 http://www.ncbi.nlm.nih.gov/pubmed/22940700. DOI: 10.1039/c2cs35208g [DOI] [PubMed] [Google Scholar]
- Schroeder WH, Anlauf KG, Barrie LA, Lu JY, Steffen A, Schneeberger DR and Berg T 1998. Arctic springtime depletion of mercury. Nature 394(6691): 331–332. ISSN 00280836 http://www.nature.com/nature/journal/v394/n6691/abs/394331a0.html. DOI: 10.1038/28530 [DOI] [Google Scholar]
- Simpson WR, Alvarez-Aviles L, Douglas TA, Sturm M and Domine F 2005. Halogens in the coastal snow pack near Barrow, Alaska: Evidence for active bromine air-snow chemistry during springtime. Geophysical Research Letters 32(4): L04811 ISSN 00948276 http://doi.wiley.com/10.1029/2004GL021748. DOI: 10.1029/2004GL021748 [DOI] [Google Scholar]
- Simpson WR, Carlson D, Hönninger G, Douglas TA, Sturm M, Perovich D and Platt U 2007a. First-year sea-ice contact predicts bromine monoxide (BrO) levels at Barrow, Alaska better than potential frost flower contact. Atmospheric Chemistry and Physics 7(3): 621–627. ISSN 1680–7324 http://www.atmos-chem-phys.net/7/621/2007/. DOI: 10.5194/acp-7-621-2007 [DOI] [Google Scholar]
- Simpson WR, von Glasow R, Riedel K, Anderson P, Ariya P, Bottenheim J, Burrows J, Carpenter LJ, Frieß U, Goodsite ME, Heard D, Hutterli M, Jacobi HW, Kaleschke L, Neff B, Plane J, Platt U, Richter A, Roscoe H, Sander R, Shepson P, Sodeau J, Steffen A, Wagner T and Wolff E 2007b. Halogens and their role in polar boundary-layer ozone depletion. Atmospheric Chemistry and Physics 7(16): 4375–4418. ISSN 1680–7324 http://www.atmos-chem-phys.net/7/4375/2007/. DOI: 10.5194/acp-7-4375-2007 [DOI] [Google Scholar]
- Sjostedt SJ and Abbatt JPD. 2008. Release of gas-phase halogens from sodium halide substrates: heterogeneous oxidation of frozen solutions and desiccated salts by hydroxyl radicals. Environmental Research Letters 3(4): 045007 ISSN 1748–9326 DOI: 10.1088/1748-9326/3/4/045007 [DOI] [Google Scholar]
- Steffen A, Douglas T, Amyot M, Ariya P, Aspmo K, Berg T, Bottenheim J, Brooks S, Cobbett F, Dastoor A, Dommergue A, Ebinghaus R, Ferrari C, Gardfeldt K, Goodsite ME, Lean D, Poulain AJ, Scherz C, Skov H, Sommar J and Temme C 2008. A synthesis of atmospheric mercury depletion event chemistry in the atmosphere and snow. Atmospheric Chemistry and Physics 8(6): 1445–1482. ISSN 1680–7324 http://www.atmos-chem-phys.net/8/1445/2008/. DOI: 10.5194/acp-8-1445-2008 [DOI] [Google Scholar]
- Stroeve J, Holland MM, Meier W, Scambos T and Serreze M 2007. Arctic sea ice decline: Faster than forecast. Geophysical Research Letters 34(9): 2007–2008. ISSN 0094–8276 http://www.agu.org/pubs/crossref/2007/2007GL029703.shtml. DOI: 10.1029/2007GL029703 [DOI] [Google Scholar]
- Sturm M, Blum JD and Simpson WR. 2016. SNACS: Snow and Ice Processes in the Deposition and Fate of Mercury in the Arctic. Arctic Data Center. https://arcticdata.io/catalog/#view/doi:10.5065/D6930R9W. DOI: 10.5065/D6930R9W [DOI] [Google Scholar]
- Theys N, Van Roozendael M, Hendrick F, Yang X, De Smedt I, Richter A, Begoin M, Errera Q, John-ston PV, Kreher K and De Mazière M 2011. Global observations of tropospheric BrO columns using GOME-2 satellite data. Atmospheric Chemistry and Physics 11(4): 1791–1811. ISSN 1680–7324 http://www.atmos-chem-phys.net/11/1791/2011/. DOI: 10.5194/acp-11-1791-2011 [DOI] [Google Scholar]
- Toom-Sauntry D and Barrie LA. 2002. Chemical composition of snowfall in the high Arctic: 1990–1994. Atmospheric Environment 36(15–16): 2683–2693. ISSN 13522310 http://linkinghub.elsevier.com/retrieve/pii/S1352231002001152. DOI: 10.1016/S1352-2310(02)00115-2 [DOI] [Google Scholar]
- Toyota K, McConnell JC, Lupu A, Neary L, McLin-den CA, Richter A, Kwok R, Semeniuk K, Kaminski JW, Gong SL, Jarosz J, Chipperfield MP and Sioris CE. 2011. Analysis of reactive bromine production and ozone depletion in the Arctic boundary layer using 3-D simulations with GEM-AQ: inference from synoptic-scale patterns. Atmospheric Chemistry and Physics 11(8): 3949–3979. ISSN 1680–7324 http://www.atmos-chem-phys.net/11/3949/2011/acp-11-3949-2011.html. DOI: 10.5194/acp-11-3949-2011 [DOI] [Google Scholar]
- Wagner T and Platt U 1998. Satellite mapping of enhanced BrO concentrations in the troposphere. Nature 395(6701): 486–490. ISSN 00280836 http://www.nature.com/doifinder/10.1038/26723. DOI: 10.1038/26723 [DOI] [Google Scholar]
- Webster MA, Rigor IG, Nghiem SV, Kurtz NT, Farrell SL, Perovich DK and Sturm M 2014. Interdecadal changes in snow depth on Arctic sea ice. Journal of Geophysical Research: Oceans. ISSN 21699275 http://doi.wiley.com/10.1002/2014JC009985. DOI: 10.1002/2014JC009985 [DOI] [Google Scholar]
- Wren SN, Donaldson DJ and Abbatt JPD. 2013. Photochemical chlorine and bromine activation from artificial saline snow. Atmospheric Chemistry and Physics 13(19): 9789–9800. ISSN 1680–7324 http://www.atmos-chem-phys.net/13/9789/2013/. DOI: 10.5194/acp-13-9789-2013 [DOI] [Google Scholar]
- Xu W, Tenuta M and Wang F 2016. Bromide and chloride distribution across the snow-sea ice-ocean interface: A comparative study between an Arctic coastal marine site and an experimental sea ice mesocosm. Journal of Geophysical Research: Oceans 121(8): 5535–5548. ISSN 21699275 http://doi.wiley.com/10.1002/2015JC011409. DOI: 10.1002/2015JC011409 [DOI] [Google Scholar]
- Yang X, Pyle JA, Cox RA, Theys N and Van Roozendael M 2010. Snow-sourced bromine and its implications for polar tropospheric ozone. Atmospheric Chemistry and Physics 10(16): 7763–7773. ISSN 1680–7324 http://www.atmos-chem-phys.net/10/7763/2010/. DOI: 10.5194/acp-10-7763-2010 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
• Figure S1. Profiles of bromide and chloride within the snowpack at 5 sites. The seawater ratio (Millero et al., 2008) is plotted for reference. DOI: https://doi.org/10.1525/elementa.352.s1
• Table S1. Sample pH, location, snowpack depth, sampling depth, and sea ice type. DOI: https://doi.org/10.1525/elementa.352.s1
• Table S2. Sample ion concentrations (μM) and uncertainties for Na+ Mg2+, Ca2+, K+, Cl−, SO42−, Br−, and NO3−. DOI: https://doi.org/10.1525/elementa.352.s1