Figure 5. Blocking miR-147b overcomes drug-tolerance.
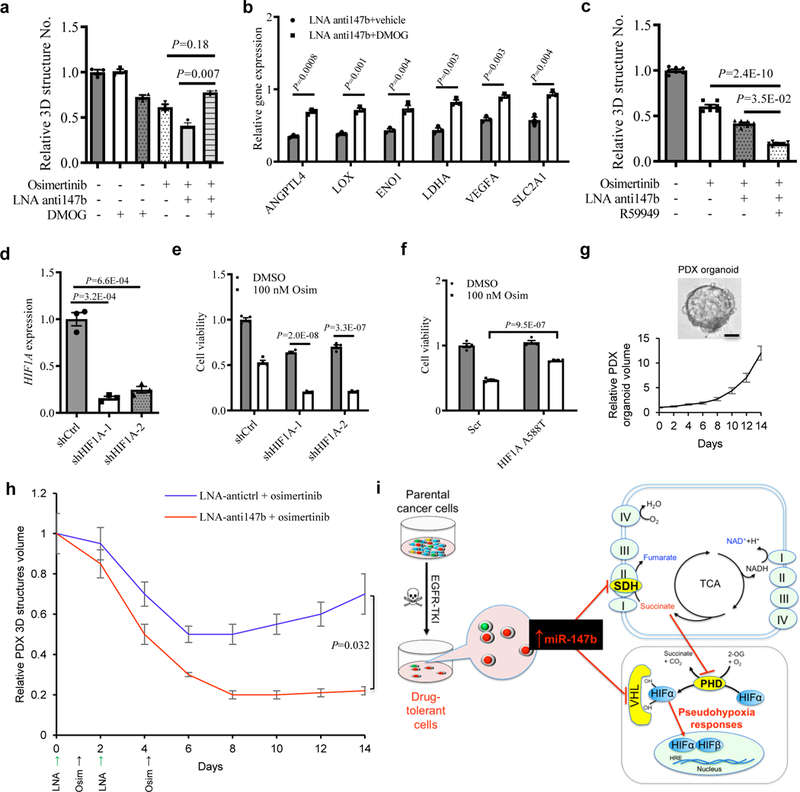
a, Fractional viability of H1975 3D structures treated with osimertinib (25 nM), LNA miR-147b inhibitor (LNA-anti147b, 90 nM), DMOG (10 μM) or combinations for 14 days. n=3 independent biological replicates.
b, qRT-PCR analysis for hypoxia gene expression in H1975 cells treated with 90 nM LNA miR-147b inhibitor (LNA-anti147b) and 10 µM DMOG or vehicle for three days. The relative gene expression in scrambled control cells treated with vehicle was calibrated as 1. n=3 independent biological replicates.
c, Fractional viability of H1975 3D structures treated with 25 nM osimertinib, 90 nM LNA-anti147b, 30 µM R59949 or combinations for 14 days. n=7 independent biological replicates.
d, qRT-PCR analysis of HIF1A in H1975 cells with shRNAs against HIF1A. H1975 cells were transfected with shRNAs against HIF1A (shHIF1A-1 and -2) or scrambled control (shCtrl) and selected with 0.5 μg/ml puromycin. GAPDH was used as endogenous control. n=3 independent biological replicates.
e, Cell viability of H1975 cells with HIF1A knockdown treated with osimertinib. The cells with shRNAs against HIF1A (shHIF1A-1 and shHIF1A-2) and scrambled control cells (shCtrl) were treated with 100 nM osimertinib or vehicle for 3 days. The cell viability was analyzed on day 4. n=4 independent biological replicates.
f, Cell viability of H1975 cells with constitutive active HIF1A mutant treated with osimertinib. The cells were transfected with HIF1A A588T and scrambled control cells (Scr) followed by 600 μg/ml neomycin selection. Then the cells were treated with 100 nM osimertinib or vehicle for 3 days. The cell viability was analyzed on day 4. n=4 independent biological replicates.
g, Derivation and growth of 3D structures from lung PDX tumors. (top) Representative phase contrast microscopy for parental EGFR mutant lung PDX-derived 3D structures in PDX_LU_10 3D structures. Repeated six times with similar results. (Bottom) growth curve of PDX 3D structures. The 3D structures size was measured every two days. The media were replenished every three days till day 14. n=3 independent biological replicates. Scale bar, 50 µm.
h, Pretreatment response on lung PDX_LU_10 3D structures with LNA miR-147b inhibitor (anti147b) and osimertinib. The 3D structures were established at medium size seven days after seeding 2000 single-cells into 3D cultures in 96-well plate. This timepoint was recorded as day 0. Then the 3D structures were administrated with LNA anti147b or antictrl (90 nM) on day 0 and day 2 or osimertinib (25 nM) on day 1 and day 4. The vehicle treated group did not receive treatments with LNA or osimertinib. The 3D structures’ size was measured every two days. The media were replenished every three days till day 14. n=3 independent biological replicates.
i, Schematic for miR-147b-driven drug-tolerance model. miR-147b is enriched in a subpopulation of parental lung cancer cells entering drug-tolerant status when they are treated with EGFR-TKIs. miR-147b mediates drug-tolerance through repressing activities of VHL and SDH leading to activated pseudohypoxia response. TKI, tyrosine kinase inhibitor; SDH, succinate dehydrogenase; TCA, tricarboxylic acid; PHD, prolyl-hydroxylase.
Data are mean ± s.e.m and were analysed with Kruskal-Wallis test (a,c); unpaired two-tailed t-test (b,d,e,f,h).
