Abstract
Background:
The relationship of internalized HIV stigma to key care cascade metrics in the United States is not well-established using large-scale, geographically diverse data.
Setting:
Center for AIDS Research Network of Integrated Clinical Systems (CNICS) cohort study
Methods:
Beginning in February 2016, we administered a yearly, validated 4-item internalized HIV stigma scale (response scale 1= strongly disagree to 5=strongly agree, Cronbach’s alpha 0.91) at seven CNICS sites and obtained cohort data through November 2017. We compared mean stigma levels by socio-demographic characteristics and used multivariable logistic regression, controlling for the same socio-demographic covariates, to evaluate the association between mean stigma and: 1) concurrent viremia; 2) missed visits; 3) poor visit constancy. We used inverse probability weighting (IPW) to account for differences between patients who did and did not undergo stigma assessment.
Results:
Of 13,183 CNICS patients, 6,448 (49%) underwent stigma assessment. Mean stigma was 1.99 (SD 1.07) and 28.6% agreed/strongly agreed with at least one stigma question. Patients <50 years, racial/ethnic minorities, cis-women, and heterosexuals had higher mean stigma. Mean stigma score was associated with concurrent viremia (AOR 1.13, 95% 1.02-1.25, p 0.02), missed visits (AOR 1.10, 95% CI 1.02-1.19, p 0.01), and poor visit constancy, though the effect on visit constancy was attenuated in the IPW model (AOR 1.05, 95% CI 0.98-1.13, p 0.17).
Conclusion:
Higher internalized HIV stigma had a modest but statistically significant association with concurrent viremia and poor retention in care. Further inquiry with prospective analyses is warranted.
Keywords: HIV stigma, virologic suppression, retention in HIV care
Introduction
Nearly thirty years into the HIV epidemic, HIV-related stigma remains a formidable barrier to care and treatment efforts for people living with HIV (PLWH). Dimensions of HIV stigma have been conceptualized as internalized (endorsement of negative feelings and beliefs about HIV), anticipated (the extent to which PLWH believe they will experience stigma as a result of HIV), or enacted (the actual experience of prejudice or discrimination because of HIV).1 Anticipated and enacted stigma relate closely to disclosure concerns, disclosure experiences, and social support, which are important but distinct factors affecting care and treatment engagement.2,3 Because some PLWH may not have disclosed their HIV status, many HIV-related stigma studies have focused on internalized HIV stigma since it is applicable to all PLWH.4 In addition to classifying different forms of HIV-related stigma, recent work has focused on delineating the mechanisms by which stigma might affect care and treatment outcomes (e.g. via affective states or interpersonal factors),5-8 along with emphasizing the important roles of structural stigma (e.g. laws or policies that disadvantage or discriminate) and intersectional stigma (e.g. stigma related to one’s race, economic circumstances, or sexual orientation).9 Indeed, a growing body of literature demonstrates that internalized HIV stigma negatively impacts antiretroviral (ART) adherence,10 in particular via increased depression8 and anxiety,5,11 lower self-efficacy,12,13 and increased social isolation.8 Internalized HIV stigma may also impact retention in HIV care and virologic suppression through similar affective, cognitive, and social mechanisms,.
While there is a possible conceptual basis for how internalized HIV stigma might relate to retention in care and virologic suppression, there is a limited and somewhat mixed evidence base on this topic and the association of internalized HIV stigma with these key cascade metrics is not well established. Few large-scale assessments exist in the United States (US) of internalized HIV stigma, and a key first step prior to the study of mechanisms by which stigma may relate to cascade outcomes is to establish a robust direct association Existing research has generally been limited by the use of single sites, lack of viral load measurements, and small sample sizes14 and demonstrated different results. In Los Angeles, PLWH with high internalized HIV stigma were more likely to report poor access to care and lack of regular care, but these effects were not observed in adjusted analyses.15 Among mostly heterosexual, racial/ethnic minority HIV patients in the Bronx, NY, internalized HIV stigma was suggestive of gaps in care along with ART non-adherence, though this result failed to reach statistical significance, likely, the authors inferred, due to limited power.16 Among a sample of mostly white men who have sex with men (MSM) in Boston, internalized HIV stigma did not predict primary care appointment attendance.17 At a safety-net HIV clinic in San Francisco, higher stigma at clinic intake predicted better retention in care at one year.18 At an academic HIV clinic in Birmingham, Alabama, internalized HIV stigma was associated with poor retention in care and ART non-adherence, but these studies did not examine viral load.5-7
Only one study to date has gathered data on internalized HIV stigma in a large sample of PLWH in the United States. As part of the Centers for Disease Control and Prevention’s Medical Monitoring Project (MMP), a surveillance system designed to collect nationally representative estimates of behavioral and clinical characteristics of PLWH receiving medical care, investigators determined the prevalence of internalized HIV stigma and examined its relationship to sustained virologic suppression, defined as all viral load measurements <200 copies/mL in the year prior to stigma assessment.19 This study found that 79% of respondents endorsed at least one stigma statement and that groups with known disparities in HIV care and treatment outcomes (e.g. youth, women, transgender individuals, racial/ethnic minorities, and heterosexuals) had higher stigma scores. Surprisingly, internalized HIV stigma was not associated with sustained virologic suppression after adjustment for age, nor was age an effect modifier of the relationship between stigma and virologic suppression. Limitations of this study were that it did not examine the role of retention in care and it was not documented whether stigma assessment was truly concurrent with viral load, i.e., viral load could have been measured at one time point up to twelve months prior to stigma assessment.
To help fill the gap in what is known about HIV-related stigma, we sought to use a unique source of data (the CFAR Network of Integrated Clinical Systems or CNICS) to describe internalized HIV stigma in a large, geographically diverse group of PLWH across the US and investigate its relationship to retention in care histories and concurrent virologic suppression. We hypothesized that the large, multi-site nature of the CNICS cohort might enable us to detect small but significant cross-sectional associations between stigma and these cascade outcomes.
Methods
Study Setting and Participants
The CNICS cohort study utilizes electronic medical (EMR) data on demographics, laboratory results, appointments, medications, comorbid conditions, and vital status for approximately 32,000 patients at eight academic HIV clinics across the US: University of Alabama, Birmingham (UAB), University of Washington (UW), University of California, San Diego (UCSD), Case Western Reserve University (CWRU), University of California, San Francisco (UCSF), Fenway Community Health Center of Harvard University (FCH), University of North Carolina at Chapel Hill (UNC), and Johns Hopkins University (JHU).20 Patient-reported outcomes (PROs), which consist of self-administered surveys, are obtained every four to six months on touch screen tablets or computers as part of routine primary care visits.21 PROs includes validated measures of ART adherence, depressive symptoms, substance use, sexual risk behavior, sexual orientation, and gender identity. Each CNICS site has institutional review board approval to collect and transmit EMR and PROs data to a coordinating center at UW, where quality checks are performed before de-identified data is provided to investigators.
Beginning in February 2016, we incorporated a validated four-item internalized HIV stigma scale (response categories 1= strongly disagree 2=disagree 3=neutral 4=agree 5= strongly agree) into PROs on a yearly basis. This assessment represented a short version of a six-item scale that was shown to have high alpha (0.87-0.89) in a similar type of clinic and correlated in the expected directions with measures of affective health and well-being;16 the scale developers calculated alpha 0.85 in their original sample with the 4-item version (Valerie Earnshaw, personal communication). A brief stigma scale was chosen to decrease burden on clinic flow, given that PROs are administered in routine care rather than in a study setting. The study population consisted of CNICS patients from the seven sites administering PROs through the time that data were uploaded to the coordinating center in November 2017 (CWRU PROs began in August 2017 and were not part of this upload). Because of site differences in stigma measurement rollout dates and data censoring prior to upload, each site had slightly different periods of stigma assessment represented in the final analysis dataset, ranging from 9 to 19 months. While our primary analyses focus on patients with stigma data, we used EMR data on the entire cohort to account for potential differences in patients who completed PROs vs. those who did not (see Statistical Analysis).
Predictor:
The primary predictor was mean stigma level (higher values = greater internalized stigma). Mean stigma levels were ascertained if at least three of four of the stigma items were answered and consisted of the mean of individual item responses.
Outcomes:
Study outcomes were: 1) concurrent viremia, defined as a viral load >200 copies/mL +/− 90 days of the stigma assessment; 2) retention in care, defined, as is standard for best practices in the field22 using a measure based on missed or “no show” primary care visits and a visit constancy measure based on kept primary care visits. Because counts of missed visits are a standard way to characterize retention,23 the missed visits measure consisted of having missed ≥2 primary care visits in the year prior to stigma assessment. Visit constancy was similarly defined using a standard method in the field as having had at least one kept primary care visit in two consecutive six-month periods,22-24 starting 90 days before the stigma assessment so as not to include the visit at which stigma was assessed in the calculation of visit constancy. One site (UNC) was excluded from the missed visit analysis because it does not currently report missed primary care visits to the CNICS data coordinating center.
Covariates:
Covariates for the main models were age, gender identity, sexual orientation, race/ethnicity, length of time in CNICS, and site.
Statistical Analysis
Descriptive statistics were used to characterize the study population. While uptake of PROs is generally high, not all patients complete it due to limited visit time, symptoms, or competing priorities, thus we compared patients completing the stigma scale to those who did not. We then calculated the distribution of responses to each stigma item, compared mean levels of stigma by socio-demographic characteristics of interest, and plotted the proportion of patients with viremia by mean stigma score.
Multivariable logistic regression models evaluated the association between mean stigma and: 1) concurrent viremia; 2) poor retention, and; 3) poor visit constancy. We assessed linearity of stigma as a continuous predictor with the log odds of outcomes using cumulative sums of residuals. For the viremia model, we only included patients with at least 180 days between CNICS enrollment and viral load measurement in order to ensure individuals had adequate time to initiate ART and achieve viral suppression. We omitted patients whose stigma assessments occurred <90 days before database closure to allows all included patients equal opportunity to have a viral load measurement. For the missed visits model, patients needed to have at least 365 days of time in CNICS and two scheduled primary care visits. For the visit constancy model, patients needed to have at least 455 days (90 +365 days) of time in CNICS. Models were adjusted for covariates of interest and site was included as a dummy variable.
While very few demographic and clinical differences were observed between patients who did and did not complete the stigma assessment, as well as between those who did not any attend scheduled primary care appointments and those who did, we repeated the complete case models for viremia and retention in care using inverse probability weighting (IPW) to account for the likelihood of having completed a stigma assessment. Weights were generated as the product of two preliminary weights, which were obtained as the inverse of the probability of completing a stigma assessment derived from two separate models. The first weight-generating model included age, present sex as defined in the EMR, race/ethnicity, length of time in CNICS, site, and viremia defined either concurrently with the stigma assessment or at the last available measurement during the time the stigma assessment was offered. The second weight-generating model included as the single predictor only the count of attended primary care visits during the interval when stigma assessments were available.
Due to a reduction of n=5,276 to a listwise n=4,269 for the complete case viremia models (19% missing data compared to 4% missing data in the retention models), we repeated all models using multiple imputation for missing viral load and covariate data as a sensitivity analysis. The imputation models were based on multiple imputation (MI) with M=50 imputations with mean stigma score passively generated as the mean of the four individual stigma questions after imputation of missing responses to those questions. All other analysis variables were included, along with the following auxiliary variables: viral load status at prior measurement, retention over the past year (≥2 missed visits vs. <2 missed visits), CD4 count +/− 90 days of stigma assessment (square-root transformed), present sex from the EMR; hepatitis C antibody status, and an indicator for whether the person had attended ≥1 primary care visit within the 6 months before the stigma assessment.
We screened for two-way interactions between mean stigma and each of the model covariates and because no evidence of interaction was found, the analyses described below include stigma and covariates as main effects. All statistical analyses were conducted in Stata v14 (StataCorp LLC, College Station, TX).
Results
Between February 2016 - November 2017, there were 13,183 CNICS patients with at least one scheduled primary care appointment, of whom 6,448 (49%) underwent stigma assessment (Figure 1). The distribution of stigma assessments across sites was as follows: UAB (26%), UCSD (20%), UW (15%), JHU (12%), UCSF (9%), FCH (9%), and UNC (9%). Median age was 49 years (IQR 39-56) with 49% ≥50 years of age; 18% identified as cis-female and 3% as a gender minority (e.g. transgender); 67% sexual minority (e.g. men who have sex with men); 41% were White, 40% were Black, and 14% were Latino, and the median time of enrollment in CNICS was 7 years (IQR 3-12). There were concurrent viral load measurements in 83% of patients, of whom 88% had viral load <200 copies/mL; 24% of patients had ≥2 missed visits in the past year and 19% did not achieve visit constancy. Patients were less likely to have a PROs stigma assessment if they were newly enrolled in CNICS during the study period (p <0.001) (there were otherwise no differences by length of time enrolled in CNICS). Patients who kept no scheduled primary care appointments (n=72) during the study period were more likely to be Black (p 0.001). The mean number of kept primary care visits was higher in those without a stigma assessment (3.3 vs 2.1, p <0.001).
Figure 1:
Flowchart of CNICS Patients
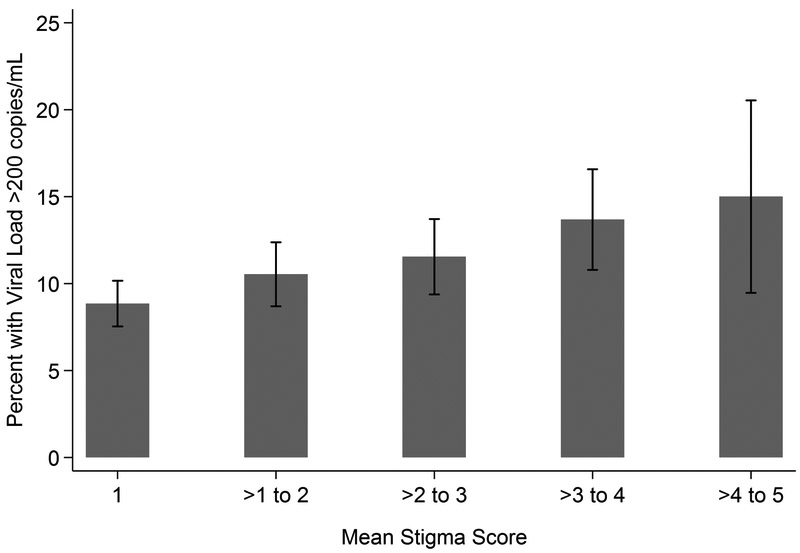
Cronbach’s alpha on the stigma scale was 0.91. The proportion of patients who agreed or strongly agreed varied by stigma question: 9.2% for “Having HIV makes me feel like a bad person,” 11.5% for “Having HIV is disgusting to me,” 17.2% for “I think less of myself because I have HIV,” and 24.9% for “I feel ashamed of having HIV” (Figure 2). Overall, 1.842 (28.6%) of patients agreed or strongly agreed with one stigma question. Mean stigma was defined for 6,401 patients on the basis of ≤1 missing item responses and was 1.99 (out of a possible score of 5, standard deviation 1.07). There were differences in mean stigma by all sociodemographic characteristics, with patients who were younger, racial/ethnic minority, cis-gender female, heterosexual, and with less time in CNICS endorsing higher levels of stigma (Table 1). In addition, the proportion of patients with concurrent viremia increased as mean stigma increased (Figure 3).
Figure 2:
Distribution of Responses to Each Stigma Question
Table 1.
Mean Stigma Score by Characteristic of Interest (n=6,401)
| Characteristic | Mean Stigma Score (SD) |
ANOVA p-value F(df num, df den) = F statistic |
|---|---|---|
| Overall | 1.99 (1.07) | |
| Age, years | ||
| 18-29 | 2.11 (1.09)a | <0.0001 |
| 30-39 | 2.07 (1.14)a | F(3,6397)=19.18 |
| 40-49 | 2.09 (1.10)a | |
| 50+ | 1.89 (1.01)b | |
| Race/Ethnicity | ||
| White | 1.94 (1.04)a | 0.035 |
| Black | 2.01 (1.09)a | F(3,6350)=2.86 |
| Latino | 2.03 (1.09)a | |
| Other | 2.07 (1.07)a | |
| Gender Identity | ||
| Cis-gender male | 1.94 (1.04)a | <0.0001 |
| Cis-gender female | 2.20 (1.16)b | F(2,6398)=27.87 |
| Gender minority | 2.08 (1.18)a | |
| Sexual Identity | ||
| Heterosexual-identified | 2.09 (1.11) | <0.0001 |
| Sexual minority | 1.92 (1.04) | F(1,6219)=37.55 |
| Years in CNICS | ||
| 0-3 years | 2.13 (1.11)a | <0.0001 |
| 4-7 years | 1.97 (1.09)b | F(3,6397)=21.91 |
| 8-12 years | 1.96 (1.07)b | |
| ≥13 years | 1.84 (0.97)c |
Pairwise comparisons of mean stigma between levels of demographic characteristics were performed separately for each comparison with the Sidak adjustment for multiple comparisons. Means with the same superscript are not significantly different.
Figure 3:
Concurrent Viremia by Mean Stigma Score
Model results using complete case analysis and multiple imputation were nearly identical, and model results using complete case analysis and complete case analysis with inverse probability weighting (IPW) were also very similar and reached identical substantive conclusions with the exception of the visit constancy model. Therefore, we present the complete case and complete case plus IPW models as our main models (Table 2). Mean stigma score (AOR 1.13, 95% CI 1.02-1.25, p 0.02) and Black race (AOR 2.23, 95% CI 1.64-3.02, p <0.0001) had statistically significant associations with concurrent viremia, as did age categories under age 50. Similar results were observed for poor retention as defined by missed visits; in addition, gender-minority identification was also associated with missed visits (AOR 1.78, 95% CI 1.04-3.05, p 0.04). In the complete case analysis, stigma was significantly associated with poor visit constancy (AOR 1.10, 95% CI 1.03-1.17), p 0.003). However, this effect was attenuated in the IPW model (AOR 1.05, 95% CI 0.98-1.13, p 0.17), where younger age and length of time in CNICS were significantly associated with poor visit constancy.
Table 2.
Adjusted Odds Ratios for the Association Between Internalized HIV Stigma and HIV Care Cascade Outcomes
| Concurrent Viremia n=4,269 |
Poor Retention (≥2 Missed Visits in Prior Year) n=4,214 |
Lack of Six-Month Visit Constancy n=5,317 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Complete Case Analysis (CCA) |
Complete Case Analysis with Inverse Probability Weights (IPW) |
Complete Case Analysis (CCA) |
Complete Case Analysis with Inverse Probability Weights (IPW) |
Complete Case Analysis (CCA) |
Complete Case Analysis with Inverse Probability Weights (IPW) |
||||||
| Adjusted Odds Ratio (95% CI) |
p- value |
Adjusted Odds Ratio (95% CI) |
p- value |
Adjusted Odds Ratio (95%CI) |
p- value |
Adjusted Odds Ratio (95% CI) |
p- value |
Adjusted Odds Ratio (95% CI) |
p- value |
Adjusted Odds Ratio (95% CI) |
p- value |
|
| Mean Stigma | 1.13 (1.03–1.24) | 0.01 | 1.13 (1.02–1.25) | 0.02 | 1.12 (1.05-1.20) | 0.001 | 1.10 (1.02–1.19) | 0.01 | 1.09 (1.02–1.17) | 0.008 | 1.05 (0.98–1.13) | 0.17 |
| Age, years | ||||||||||||
| 18-29 | 2.13 (1.42–3.21) | <0.001 | 1.89 (1.23–2.91) | 0.004 | 1.95 (1.40–2.72) | 0.0001 | 1.73 (1.20–2.48) | 0.003 | 1.88(1.36–2.59) | 0.0001 | 1.76 (1.25–2.49) | 0.0012 |
| 30-39 | 1.91 (1.43–2.54) | <0.0001 | 1.706 (1.24–2.34) | 0.001 | 2.17 (1.76–2.68) | <0.0001 | 2.15 (1.69–2.74) | <0.0001 | 2.10 (1.72–2.56) | <0.0001 | 2.21 (1.77–2.76) | <0.0001 |
| 40-49 | 1.76 (1.37–2.26) | <0.0001 | 1.629 (1.24–2.14) | <0.001 | 1.67 (1.40–2.00) | <0.0001 | 1.65 (1.35–2.01) | <0.0001 | 1.45 (1.22–1.73) | <0.0001 | 1.39(1.16–1.68) | 0.0005 |
| ≥50 | Reference | -- | Reference | -- | Reference | -- | Reference | -- | Reference | -- | Reference | -- |
| Race/Ethnicity | ||||||||||||
| Black | 2.24 (1.71–2.94) | <0.0001 | 2.23 (1.64–3.02) | <0.0001 | 1.97 (1.60–2.42) | <0.0001 | 2.14 (1.68–2.72) | <0.0001 | 0.95(0.80–1.14) | 0.61 | 0.93 (0.77–1.12) | 0.42 |
| Latino | 0.97 (0.68–1.38) | 0.86 | 0.88 (0.60–1.3) | 0.51 | 1.07 (0.85–1.34) | 0.56 | 1.12 (0.86–1.45) | 0.40 | 0.79 (0.62–1.00) | 0.05 | 0.81 (0.62–1.05) | 0.10 |
| Other | 1.39 (0.85–2.28) | 0.19 | 1.17 (0.68–2.01) | 0.57 | 0.83 (0.58–1.21) | 0.34 | 0.88 (0.58–1.32) | 0.53 | 0.91 (0.64–1.30) | 0.62 | 0.96 (0.64–1.43) | 0.83 |
| White | Reference | -- | Reference | -- | Reference | -- | Reference | -- | ||||
| Gender Identity | ||||||||||||
| Cis-female | 1.14 (0.84–1.54) | 0.40 | 1.20 (0.85–1.69) | 0.30 | 1.08 (0.86–1.36) | 0.51 | 1.05 (0.81–1.37) | 0.70 | 0.80(0.64–1.00) | 0.05 | 0.80 (0.63–1.01) | 0.06 |
| Gender minority | 1.12 (0.62–2.02) | 0.71 | 0.86 (0.41–1.80) | 0.69 | 2.21 (1.46–3.34) | 0.0002 | 1.78 (1.04–3.05) | 0.04 | 1.07(0.68–1.69) | 0.77 | 1.30 (0.77–2.20) | 0.33 |
| Cis-male | Reference | -- | Reference | -- | Reference | -- | Reference | -- | ||||
| Sexual Identity | ||||||||||||
| Heterosexual | 0.95 (0.72–1.24) | 0.69 | 0.88 (0.65–1.19) | 0.40 | 1.12 (0.91–1.37) | 0.30 | 1.04 (0.82–1.33) | 0.73 | 1.24 (1.02–1.50) | 0.03 | 1.20 (0.97–1.48) | 0.09 |
| Sexual Minority | Reference | -- | Reference | — | Reference | -- | Reference | -- | ||||
| Years in CNICS | 1.01 (0.99–1.03) | 0.29 | 1.01 (0.99–1.03) | 0.46 | 0.99 (0.97–1.00) | 0.15 | 0.99 (0.98–1.01) | 0.32 | 1.03(1.01–1.04) | 0.0002 | 1.02 (1.01-1.04) | 0.003 |
Note: All models were also adjusted for site, but results of this dummy variable are not shown for clarity.
No statistically significant interactions were found between mean stigma and each of the model covariates (all interaction p values were > 0.05).
Discussion
In a large, geographically diverse cohort of individuals in HIV care, about one-quarter of patients agreed or strongly agreed with at least one question of a four-item internalized HIV stigma scale and overall mean levels of internalized HIV stigma were not high (approximately 2 on a scale of 1-5). However, levels were higher among groups previously described in the literature as more affected by HIV-related stigma, such as adults under age 50, racial/ethnic minorities, those who identified as cis-gender female, and those who identified as heterosexual. 19 In adjusted analyses, each unit increase in internalized HIV stigma increased the odds of a history of missed visits and concurrent viremia by 10%-15% while a smaller effect was observed on retrospective six-month visit constancy. Therefore, it appears that even low levels of stigma are associated with care cascade outcomes. While younger age was significantly associated with poor outcomes in all models, we found no evidence of interaction between stigma and age, nor did we find interactions with other covariates, suggesting no differential association of stigma with any of the three clinical outcomes we assessed by these covariates.
Our findings differ from those of the MMP, which found that stigma was no longer associated with viral load after adjusting for age. Reasons for this difference may be that we assessed viral load in the 90 days bracketing the stigma assessment, rather than over the year prior to stigma assessment as done in the MMP. In addition, the MMP used a stigma scale in which two of the items were about disclosure concerns, rather than negative self-attitudes.25 A recently published analysis of enacted and internalized HIV stigma in Black women in HIV care in Chicago and Birmingham, Alabama, found a modest cross-sectional association with viral load similar to our study,26 however, a subsequent longitudinal analysis from the same study found that when the enacted and internalized HIV stigma sub-scales were evaluated as separate predictors, internalized stigma was not associated with viral suppression.27 It is worth noting that younger age and Black race had higher odds ratios than stigma in the viremia and missed visits models, which may mean that there other variables outside of stigma, particularly structural or psychosocial factors, that relate to clinical outcomes for these groups.
The lack of a statistically significant association between stigma and poor visit constancy in the complete case plus IPW model (as opposed to the complete case model without IPW) may reflect the fact that IPW is an inefficient estimator or it may mean that stigma is not strongly correlated with six-month visit constancy. Indeed, the relationship between internalized stigma and visit attendance may be complex. Some individuals may avoid the clinic due to disclosure concerns, but in other cases the clinic may be the only place where individuals have disclosed their HIV status and can obtain HIV-related support.
What our study contributes to the literature are established cross-sectional associations between internalized HIV stigma and the care cascade outcomes of retention in care and viral suppression, the next step is to assess potential mediators/moderators of these relationships, such as social support, depression, substance use, and antiretroviral adherence. These analyses are best conducted with longitudinal rather than cross-sectional data to better infer causal relationships.14 Given ongoing collection of viral load and appointment data, the CNICS cohort structure will enable future investigation of these behavioral variables using the most robust analytic approaches.
There are limitations and strengths to our analysis. While our analyses are cross-sectional and we cannot infer causality, we provide the first large-scale assessment of internalized stigma and retention in HIV care in the U.S. In addition, we contribute an examination of the concurrent (rather than retrospective) relationship between stigma and viral load. Although we assessed only one form of HIV-related stigma, we used a validated scale.16 Our analysis may be limited by not being able to include other unmeasured confounders of the relationship between stigma and cascade outcomes. Another limitation of this analysis is that it was restricted only to individuals accessing HIV care. However, a strength of our study is the relatively long time over which stigma was assessed (9-19 months) because even patients tenuously connected to care should have had an opportunity to complete the PROs survey. Moreover, if patients with high stigma are truly out of care then we are underestimating the association between stigma and HIV are outcomes. We also attempted to address differences between those who completed a stigma assessment and those who did not by using inverse probability weighting, thus addressing potential concerns related to sampling bias and lack of generalizability to the clinic sites. We also employed multiple imputation as a check on the influence of missing data on our observed findings and found very similar results to our complete case models.
In sum, we found that levels of internalized HIV stigma among a cohort of U.S. patient in routine care were not high but that stigma was modestly but significantly associated with concurrent viremia and missed primary care visits, which are known in the literature to predict subsequent mortality.28,29 These findings suggest that efforts to end the HIV epidemic must continue to acknowledge the crucial role of stigma and better understand the mechanisms through which it relates to HIV care cascade outcomes.
Acknowledgments:
This research was made possible by National Institutes of Health R01 MH102198-S1. We would like to thank Valerie Earnshaw and Laramie Smith for the four-item version of their six-item scale.
Conflicts of Interest and Sources of Funding: None related to this manuscript. Dr. Christopoulos has received investigator-initiated grant support from Gilead Sciences and has served as a community advisory board member for Gilead. This work was supported by National Institutes of Health R01 MH102198-S1 and R24 AI067039.
Footnotes
Previous Presentation: Presented in part at the 25th Conference on Opportunistic Infections and Retroviruses; March 4-7, 2018; Boston, Massachusetts.
References
- 1.Earnshaw VA, Chaudoir SR. From conceptualizing to measuring HIV stigma: a review of HIV stigma mechanism measures. AIDS and behavior. 2009;13(6):1160–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wohl AR, Galvan FH, Myers HF, et al. Do social support, stress, disclosure and stigma influence retention in HIV care for Latino and African American men who have sex with men and women? AIDS and behavior. 2011;15(6):1098–1110. [DOI] [PubMed] [Google Scholar]
- 3.Kelly JD, Hartman C, Graham J, Kallen MA, Giordano TP. Social support as a predictor of early diagnosis, linkage, retention, and adherence to HIV care: results from the steps study. J Assoc Nurses AIDS Care. 2014;25(5):405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kipp AM, Audet CM, Earnshaw VA, Owens J, McGowan CC, Wallston KA. Revalidation of the Van Rie HIV/AIDS-related stigma scale for use with people living with HIV in the United States. PLoS One. 2015;10(3):e0118836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blake Helms C, Turan JM, Atkins G, et al. Interpersonal Mechanisms Contributing to the Association Between HIV-Related Internalized Stigma and Medication Adherence. AIDS Behav. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turan B, Budhwani H, Fazeli PL, et al. How Does Stigma Affect People Living with HIV? The Mediating Roles of Internalized and Anticipated HIV Stigma in the Effects of Perceived Community Stigma on Health and Psychosocial Outcomes. AIDS Behav. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rice WS, Crockett KB, Mugavero MJ, Raper JL, Atkins GC, Turan B. Association Between Internalized HIV-Related Stigma and HIV Care Visit Adherence. J Acquir Immune Defic Syndr. 2017;76(5):482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turan B, Smith W, Cohen MH, et al. Mechanisms for the Negative Effects of Internalized HIV-Related Stigma on Antiretroviral Therapy Adherence in Women: The Mediating Roles of Social Isolation and Depression. J Acquir Immune Defic Syndr. 2016;72(2):198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turan B, Hatcher AM, Weiser SD, Johnson MO, Rice WS, Turan JM. Framing Mechanisms Linking HIV-Related Stigma, Adherence to Treatment, and Health Outcomes. Am J Public Health. 2017;107(6):863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc. 2013;16(3 Suppl 2): 18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rueda S, Mitra S, Chen S, et al. Examining the associations between HIV-related stigma and health outcomes in people living with HIV/AIDS: a series of meta-analyses. BMJ Open. 2016;6(7):e011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diiorio C, McCarty F, Depadilla L, et al. Adherence to antiretroviral medication regimens: a test of a psychosocial model. AIDS Behav. 2009;13(1):10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seghatol-Eslami VC, Dark HE, Raper JL, Mugavero MJ, Turan JM, Turan B. Brief Report: Interpersonal and Intrapersonal Factors as Parallel Independent Mediators in the Association Between Internalized HIV Stigma and ART Adherence. J Acquir Immune Defic Syndr. 2017;74(1):e18–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sweeney SM, Vanable PA. The Association of HIV-Related Stigma to HIV Medication Adherence: A Systematic Review and Synthesis of the Literature. AIDS Behav. 2016;20(1):29–50. [DOI] [PubMed] [Google Scholar]
- 15.Sayles JN, Wong MD, Kinsler JJ, Martins D, Cunningham WE. The association of stigma with self-reported access to medical care and antiretroviral therapy adherence in persons living with HIV/AIDS. Journal of general internal medicine. 2009;24(10):1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Earnshaw VA, Smith LR, Chaudoir SR, Amico KR, Copenhaver MM. HIV stigma mechanisms and well-being among PLWH: a test of the HIV stigma framework. AIDS and behavior. 2013;17(5):1785–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Traeger L, O'Cleirigh C, Skeer MR, Mayer KH, Safren SA. Risk factors for missed HIV primary care visits among men who have sex with men. J Behav Med. 2012;35(5):548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christopoulos KA, Johnson MO, Dilworth S, Neilands TB, Gandhi M, Geng E. Higher Levels of Internalized HIV Stigma at Clinic Intake Paradoxically Predict More Successful Linkage to Primary Care. 11th International Conference on HIV Treatment and Prevention Adherence, May 9-11, 2016, Fort Lauderdale. [Google Scholar]
- 19.Baugher A, Beer L, Fagan J, Mattson C, Freedman M. Skarbinski J. Internalized Stigma in a Population-Based Sample of US HIV-Infected Adults in Care. Paper presented at: Conference on Retroviruses and Opportunistic Infections 2015; Seattle, WA. [Google Scholar]
- 20.Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol. 2008;37(5):948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozak MS, Mugavero MJ, Ye J, et al. Patient reported outcomes in routine care: advancing data capture for HIV cohort research. Clin Infect Dis. 2012;54(1):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mugavero MJ, Westfall AO, Zinski A, et al. Measuring retention in HIV care: the elusive gold standard. Journal of acquired immune deficiency syndromes. 2012;61(5):574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mugavero MJ, Davila JA, Nevin CR, Giordano TP. From access to engagement: measuring retention in outpatient HIV clinical care. AIDS Patient Care STDS. 2010;24(10):607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crawford TN, Sanderson WT, Thornton A. A comparison study of methods for measuring retention in HIV medical care. AIDS Behav. 2013;17(9):3145–3151. [DOI] [PubMed] [Google Scholar]
- 25.Kalichman SC, Simbayi LC, Cloete A, Mthembu PP, Mkhonta RN, Ginindza T. Measuring AIDS stigmas in people living with HIV/AIDS: the Internalized AIDS-Related Stigma Scale. AIDS Care. 2009;21(1):87–93. [DOI] [PubMed] [Google Scholar]
- 26.Lipira L, Williams EC, Huh D, et al. HIV-Related Stigma and Viral Suppression Among African-American Women: Exploring the Mediating Roles of Depression and ART Nonadherence. AIDS Behav. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kemp CG, Lipira LL, Huh D, et al. HIV stigma and viral load among African-American women receiving treatment for HIV: A longitudinal analysis. AIDS. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mugavero MJ, Lin HY, Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48(2):248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mugavero MJ, Westfall AO, Cole SR, et al. Beyond core indicators of retention in HIV care: missed clinic visits are independently associated with all-cause mortality. Clin Infect Dis. 2014;59(10):1471–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]