Abstract
Introduction:
Previous biomedical studies identified many lifestyle exposures that could possibly represent risk factors for dementia in general or dementia due to Alzheimer’s disease (AD). These lifestyle exposures are mainly mentioned in free-text electronic health records (EHRs). However, automatic extraction and assessment of these exposures using EHRs remains understudied.
Methods:
A natural language processing (NLP) approach was adopted to extract lifestyle exposures and intervention strategies from the clinical notes of 260 patients with clinical diagnoses of AD dementia and 260 age-matched cognitively unimpaired persons. Statistics of lifestyle exposures were compared between these two groups. The mapping results of the NLP extraction were evaluated by comparing the results with data captured independently by clinicians.
Results:
Thirty out of fifty-five potentially relevant lifestyle exposures were mentioned in our clinical note dataset. Twenty-two dietary factors and three substance abuses that were potentially relevant were not found in clinical notes. Patients with AD dementia were significantly exposed to more of the potential risk factors compared to the cognitively unimpaired subjects (χ2 = 120.31, p-value < 0.001). The average accuracy of the automated extraction was 74.0% in comparison with the manual review of randomly selected 50 sample documents.
Discussion and conclusion:
We illustrated the feasibility of NLP techniques for the automated evaluation of a large number lifestyle habits using free-text EHR data. We found that AD dementia patients were exposed to more of the potential risk factors than the comparison group. Our results also demonstrated the feasibility and accuracy of investigating putative risk factors using NLP techniques.
Keywords: Alzheimer’s disease, Electronic health records, Natural language processing, Lifestyle exposure
1. Introduction
Alzheimer’s disease (AD) is a degenerative brain disease and the most common form of dementia in the United States [1]. Alzheimer’s disease affects about 5.7 million Americans and is growing exponentially with an estimate of 13.8 million being affected by midcentury [2]. Since the number of patients with AD is expected to grow, finding ways to prevent and lower the risk of AD is crucial [3]. The majority of investigations into potential lifestyle-related risk factors for AD dementia are retrospective cross-sectional analyses based on selfreport questionnaires [4]. However, questionnaire assessment is limited to patient’s subjective experience, which is not suitable for objective measurements such as laboratory test results [5]. Moreover, many questionnaires investigate the exposure factors, such as food intake over one year instead of daily records or 24 -h recalls, resulting in an increased recall bias [6]. Furthermore, the exposures studied in questionnaire surveys are not scalable. Table 1 lists the recent lifestyle risk factor studies [7-11] of AD, in which only 1–3 exposures were investigated in each survey, highlighting the need for a more comprehensive approach which would produce more valid and meaningful results.
Table 1.
Cross-sectional lifestyle studies of Alzheimer’s disease.
| Study | Study Design | Location | Sample Size | Lifestyle Exposures Investigated | No. of Exposures |
|---|---|---|---|---|---|
| Mosconi L, et al (2018) | Cross-sectional | USA | 116 | Mediterranean diet Physical activity Intellectual activity |
3 |
| Berti V, et al, (2015) | Cross-sectional | USA | 52 | Nutrition pattern | 1 |
| Gardener S, et al (2012) | Case-control | Australia | 247 cases, 723 controls | Mediterranean diet | 1 |
| Gauthier E, et al (2001) | Case-control | Canada | 68 cases, 68 controls | Pesticide exposure | 1 |
| Rogers MA, et al (1999) | Case-control | USA | 23 cases, 23 controls | Aluminum intake | 1 |
The growing availability of electronic health records (EHRs) provides an increased opportunity for a thorough investigation of risk factors for AD dementia. EHRs refer to the comprehensive records of a patient health care history that resides in digital format [12,13]. Clinical notes are free-text EHRs that contain textual descriptions of physician-patient encounters and capture the information that the author intended to collect concerning a certain medical topic, offering valuable resources for identifying lifestyle exposures that physicians believed to be clinically important [14,15]. However, since clinical notes are free-text narratives lacking a standardized structure, searching for simple keywords may result in low sensitivity [16,17]. Natural language processing (NLP) offers a solution for clinical notes processing. NLP is a field of computational techniques that allows computers to extract relevant information from human language [18] and offers a viable solution for effectively processing clinical notes. It has been widely utilized in clinical applications, such as quality measurement of laboratory tests [19], early diagnosis of disease [20], and suicide behavior screening [21]; however, to best of our knowledge, none of the previous NLP approaches have been applied to lifestyle investigation.
This study sought to apply NLP techniques to automatically extract and assess lifestyle exposures as well as corresponding intervention strategies from the EHRs of AD patients and non-demented controls. Our study was motivated by the needs to: 1) demonstrate the feasibility of NLP techniques in lifestyle investigation for AD patients; 2) determine whether previously identified lifestyle exposures were recognized in primary care settings; and 3) determine whether lifestyle interventions were delivered by primary care providers. Our dataset of clinical notes has been an invaluable resource to allow examination of lifestyle assessments and management among AD patients in primary health care settings.
2. Methods
2.1. Study subjects
The patient cohort was filtered from the Mayo Clinic Employee and Community Health (ECH), a department which delivers primary care to 140,000 patients who reside within the area surrounding Rochester, Minnesota [22]. The inclusion criteria of the cohort were: 1) a patient who received the primary care in Mayo Clinic, Rochester from 1998 to 2015; 2) a patient whose EHR data was available; 3) the presence of research authorization for using medical records for research. Patients were considered to have AD dementia based on being assigned ICD-9-CM code 331.0 (Alzheimer’s disease). A control group of patients were randomly selected from the ECH cohort who matched the average age of the AD group and did not have any ICD diagnosis code for AD or dementia. A total of 260 AD dementia patients (mean age = 82.28 ± 9.47) and 260 age-matched controls (mean age = 81.05 ± 7.80) were identified. All of their clinical notes (64,672 in total) were extracted from the clinical data warehouse and the documents of each subject were merged into a single clinical note for further analysis. This study was approved by the Institutional Review Board (IRB) for human subject research.
2.2. Study design
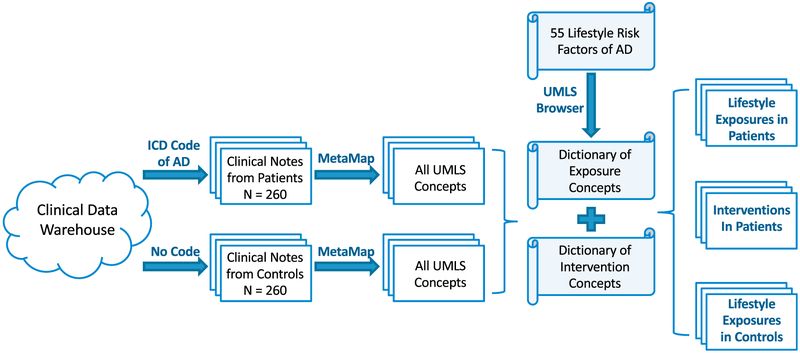
This case-control study aims to investigate the prevalence of lifestyle risk factor exposures among AD dementia patients in primary care settings. A schematic diagram of the study is presented in Fig. 1. First, we retrieved clinical notes of AD dementia patients and age-matched controls. Processing clinical notes requires tools that identify standard medical concepts from free-form text [23]. MetaMap (https://metamap.nlm.nih.gov/) is such a tool that automatically maps biomedical texts to standard medical concepts in the Unified Medical Language System (UMLS) [24-27]. MetaMap shows comparable or better performances to other mapping tools such as clinical Text Analysis and Knowledge Extraction System (cTAKES) [28,29]. We chose MetaMap since it is simpler to implement than cTAKES for people without a Java programming background.
Fig. 1.
Procedure for retrieving lifestyle information from clinical notes.
We utilized 55 lifestyle risk factors that have been identified by Kostoffa et al (Table S1) [30] as potentially being related to the development of AD dementia. These factors were retrieved from the AD-related literature consisting of 100,000 Medline abstracts and 99,610 PubMed articles. Then we collected all UMLS concepts related to each risk factor (e.g., vitamin B deficiency) and its corresponding intervention strategy (e.g., vitamin B supplement) by using online UMLS Terminology Services (default settings) as the searching browser (https://www.nlm.nih.gov/research/umls/). A UMLS concept dictionary of lifestyle exposures and a dictionary of interventions were established (described below). By comparing UMLS concepts extracted from clinical notes with two dictionaries, we can identify whether the lifestyle exposures as well as intervention strategies occurred in clinical notes.
2.3. Dictionary of lifestyle exposures
To construct the dictionary, we searched for all of the UMLS concepts corresponding to lifestyle exposure terms from UMLS browser. Searching keywords of each exposure factor were determined as following: 1) for excessive/deficient dietary factors, searching keywords are the same as the factors (e.g., “high fat diet” are the keywords for high fat diet); 2) for food additives, searching keywords are “exposure factor + diet” (e.g., “Menadione diet” for the factor Menadione); 3) for substance abuse, keywords are “exposure factors + abuse” (e.g., “amphetamine abuse” for the factor amphetamine). All searching keywords were shown in the appendix Table S1.
2.4. Dictionary of intervention strategies
To construct the dictionary, we searched for all of the UMLS concepts corresponding to lifestyle intervention terms from UMLS browser. Searching keywords were determined as following: 1) for excessive dietary factors such as high ** diet, searching keywords are “low ** diet” (e.g., “low fat diet” are the keywords for high fat diet); 2) for dietary deficiencies such as ** deficiency, we search “** supplement” (e.g., “vitamin B supplement” are the keywords for vitamin B deficiency); 3) “low + exposure factor + diet” are the keywords for food additives (e.g., “low cysteine diet” are the keywords for cysteine); 4) intervention keywords for sleep disorder include all benzodiazepine drugs that are marketed in USA, which are presently the most frequently prescribed hypnotics [31].
2.5. Extracting lifestyle exposures and interventions using NLP
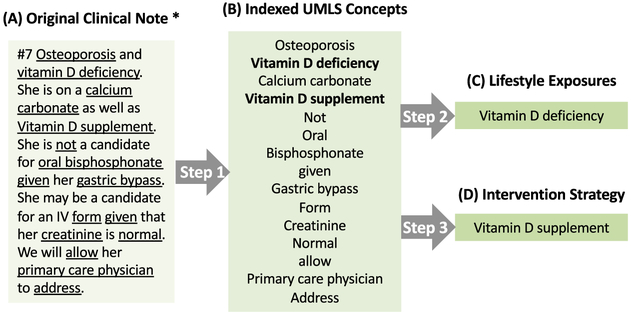
NLP is intended to take the place of manual chart review and extract information from texts automatically. There are three steps in the process, as shown in Fig. 2.
Fig. 2.
Extracting lifestyle exposures and modifications from clinical notes. * This is not an AD section. Step 1: indexing UMLS concepts from original clinical notes. Step 2: identifying lifestyle exposures. Step 3: identifying lifestyle intervention strategies.
Step 1: Mapping clinical notes to UMLS concepts.
We applied the default setting of MetaMap Windows version to index UMLS concepts from clinical notes (n = 260 in each group). Fuzzy mapping was not applied in our approach since fuzzy mapping has the potential to cause many unrelated mapping results.
Step 2: Extracting lifestyle exposures from clinical notes.
We investigated the occurrence of lifestyle exposure-related UMLS concepts among mapped clinical notes. For factors mentioned in clinical notes, number of records (denoted as m), number of patients (denoted as n) and percentage of patients were recorded. Number of records was defined as the total frequency of the certain exposure in 260 clinical notes. Number of patients was defined as the number of clinical notes that contained the specific exposure. Percentage of patients = number of patients/260. In addition, the total number of exposures was defined as how many different lifestyle exposures each patient had.
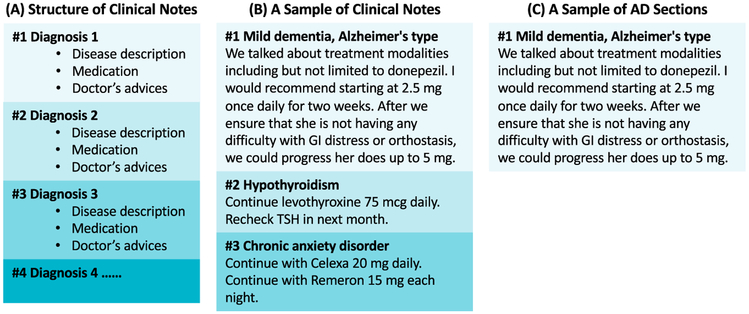
Step 3: Extracting lifestyle interventions strategies from AD sections and the complete clinical notes.
When extracting intervention strategies from clinical notes, we aimed to figure out whether these strategies were related to AD or other diseases. We used the Python NLTK package to filter clinical note sections that only record AD-related information only. As shown in Fig. 3, for patients with multiple diseases, each diagnosis and the related medical information (such as disease descriptions, medications, doctors’ advices, etc.) were recorded separately. Medical records following the diagnosis of AD were considered as AD sections. Intervention strategies in AD sections were extracted, and reflected physicians' attitudes and practices toward prevention and treatment of AD. Next, we expanded our searching range to the whole corpus of clinical notes and extracted all lifestyle interventions. Number of exposures, number of patients, and percentage of patients were recorded.
Fig. 3.
Illustration of extracting AD-related sections from a synthetic clinical note.
2.6. Limited evaluation of the NLP method in MetaMap
We randomly sampled 50 documents from the dataset for manual review. Both lifestyle risk factors and intervention strategies were manually annotated by investigators and this method was considered as the “gold standard”. Because this study does not focus on the evaluation of NLP methods in MetaMap, when MetaMap found a matching UMLS map, we manually evaluated how accurate the mapping was. Findings from the automated algorithm were compared with the gold standard, and the precision of the NLP method was calculated.
2.7. Statistical analysis
All 520 subjects were included in the statistical analysis. We compared the demographic characteristics between AD dementia patients and control subjects using Pearson’s χ2 test for categorical variables and Student’s t-test for continuous variables. Generalized linear models were fit to obtain the odds ratio (OR) and 95% confidence intervals of the top 10 exposure factors that occurred in clinical notes. All statistical analyses were performed using R statistical software (version 3.5.1, R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Study cohort demographics
The study sample consisted of 260 AD dementia patients and 260 controls from a general primary care population. Their demographics are summarized in Table 2. There was no significant difference in age, sex, race, and marital status found between AD dementia patients and the controls. Compared with the general population in the United States, this study cohort had a higher proportion of white race in both groups (97.3% in AD group, 99.2% in control group vs. 62.0% in the general population) with a correspondingly lower proportion in other races.
Table 2.
Demographic Characteristics of the Study Cohort.
| Demographics | Count(%) | Statistic | P-value | ||
|---|---|---|---|---|---|
| AD group (n = 260) |
Control group (n = 260) |
||||
| Age | < 75 | 48 (18.4) | 69 (26.5) | t = 0.625 | 0.532 |
| 76 – 85 | 111 (42.7) | 104 (40.0) | |||
| 86 – 95 | 92 (35.4) | 74 (28.5) | |||
| > 95 | 9 (3.5) | 13 (5.0) | |||
| Mean (SD) | 82.28 (9.47) | 81.05 (7.80) | |||
| Sex | Female | 127 (48.9) | 136 | χ2 = 0.492 | 0.483 |
| Male | 133 (51.1) | 124 | |||
| Race | White | 253 (97.3) | 256 (98.5) | χ2 = 5.174 | 0.160 |
| Black | 0 (0.0) | 1 (0.3) | |||
| Asian | 4 (1.5) | 0 (0.0) | |||
| Other | 3 (1.2) | 3 (1.2) | |||
| Marital status | Married | 193 (74.2) | 173 (66.5) | χ2 = 4.634 | 0.201 |
| Single | 8 (3.1) | 14 (5.4) | |||
| Divorced | 14 (5.4) | 21 (8.1) | |||
| Widowed | 45 (17.3) | 52 (20.0) | |||
3.2. Lifestyle exposures in AD patients
Using NLP techniques, we identified 20 out of 55 lifestyle risk factor exposures from clinical notes among patients with AD dementia, which could be categorized as dietary factors, daily activity and substance abuse (Table 3). Overall, tobacco smoking and malnutrition were the most common exposure factors among AD patients, affecting 145 and 134 patients respectively. 7 categories of vitamin or mineral deficiencies (including vitamin B/D/E deficiency and potassium/iron/magnesium/calcium deficiency) were also identified in our cohort. Conversely, cardiovascular/metabolic exposures, such as high fat diet, high calorie diet, high carbohydrate diet, etc., were relatively rare in this cohort (n < 3).
Table 3.
Lifestyle Exposures Reported in AD Group.
| Category | Lifestyle Exposures | No. of records |
No. of patients |
% of patients |
|---|---|---|---|---|
| Dietary Factor | High Calorie Diet | 1 | 1 | < 1.0 |
| High Carbohydrate Diet | 1 | 1 | < 1.0 | |
| High Meat Diet | 6 | 2 | < 1.0 | |
| High Fat Diet | 36 | 3 | 1.2 | |
| High Pickle Diet | 3 | 2 | < 1.0 | |
| Vitamin B Deficiency | 1181 | 28 | 10.8 | |
| Vitamin D Deficiency | 2629 | 29 | 11.2 | |
| Vitamin E Deficiency | 10 | 1 | < 1.0 | |
| Potassium Deficiency | 1454 | 43 | 16.5 | |
| Iron Deficiency | 1872 | 25 | 9.6 | |
| Magnesium Deficiency | 382 | 12 | 4.6 | |
| Calcium Deficiency | 558 | 38 | 14.6 | |
| Starvation | 22 | 2 | < 1.0 | |
| Dehydration | 1932 | 91 | 35 | |
| Malnutrition | 3567 | 134 | 51.5 | |
| Daily Activity | Physical Inactivity | 6 | 4 | 1.5 |
| Sleep Disorder | 25 | 5 | 1.9 | |
| Substance Abuse | Phencyclidine | 24 | 2 | < 1.0 |
| Tobacco Smoking | 1081 | 145 | 55.8 | |
| Excess Alcohol | 7469 | 52 | 20 |
3.3. Lifestyle interventions in AD dementia patients
Lifestyle intervention strategies retrieved from AD sections and whole clinical notes are compared in Table 4. The intervention records were very rare in AD sections with only five intervention strategies being suggested by physicians, such as physical activity (n = 123), cognitive activity (n = 45), dietary supplement (n = 19), low salt diet (n = 1) and benzodiazepines (n = 1). However, when expanding our searching range to the whole clinical notes, a total of 23 lifestyle intervention strategies were identified. Physical activity was the most commonly identified lifestyle intervention suggested by physicians (n = 227), followed by dietary supplement (n = 148), smoking sessation (n = 137), fish oil supplement (n = 94) and vegetables supplement (n = 89). In addition, vitamins (including vitamin B, C, D, and E) and mineral supplements (including potassium, iron, zinc, magnesium and calcium) were widely encouraged by physicians.
Table 4.
Lifestyle Interventions Strategies in AD Group.
| Category | Lifestyle Intervention | All Sections | AD Sections | ||||
|---|---|---|---|---|---|---|---|
| No. of Record | No. of Patient | % of Patient | No. of Record | No. of Patient | % of Patient | ||
| Dietary Factor | Low Fat Diet | 187 | 26 | 10.0 | |||
| Low Salt Diet | 379 | 51 | 19.6 | 1 | 1 | < 1.0 | |
| Low Carbohydrate Diet | 1 | 1 | < 1.0 | ||||
| Low Calorie Diet | 1 | 1 | < 1.0 | ||||
| Low Cholesterol Diet | 200 | 23 | 8.8 | ||||
| Vitamin B Supplement | 4 | 2 | < 1.0 | ||||
| Vitamin C Supplement | 4 | 1 | < 1.0 | ||||
| Vitamin D Supplement | 226 | 21 | 8.1 | ||||
| Vitamin E Supplement | 1163 | 4 | 1.5 | ||||
| Potassium Supplement | 62 | 15 | 5.8 | ||||
| Iron Supplement | 36 | 10 | 3.8 | ||||
| Zinc Supplement | 4 | 1 | < 1.0 | ||||
| Magnesium Supplement | 7 | 2 | < 1.0 | ||||
| Calcium Supplement | 381 | 38 | 14.6 | ||||
| Dietary Supplement | 1843 | 148 | 56.9 | 33 | 4 | 1.5 | |
| Nutrition Supplement | 78 | 16 | 6.2 | ||||
| Docosahexaenoic Acid | 303 | 6 | 2.3 | ||||
| Vegetables | 3141 | 89 | 34.2 | ||||
| Fatty Fish/Fish Oil | 16528 | 94 | 36.2 | 1 | 1 | < 1.0 | |
| Daily Activity | Physical Activity | 18579 | 227 | 87.3 | 123 | 23 | 8.8 |
| Cognitive Activity | 238 | 46 | 17.7 | 45 | 7 | 2.7 | |
| Benzodiazepines | 2672 | 17 | 6.5 | 19 | 1 | < 1.0 | |
| Substance Abuse | Smoking Cessation | 1200 | 137 | 52.7 | |||
3.4. Lifestyle exposures between AD and the control groups
We identified 13 lifestyle exposures from the clinical notes of the control group, (Table 5) with most exposures (11 of 13, except vitamin K deficiency and high iron diet) being found in the AD group. Individual effects and overall effects of lifestyle exposures were investigated between AD patients and non-demented controls.
Table 5.
Lifestyle Exposures Reported in Control Group.
| Category | Lifestyle Exposures | No. of Records | No. of Patients | % of Patients |
|---|---|---|---|---|
| Dietary Factor | High Iron Diet | 1 | 1 | < 1.0 |
| Vitamin B Deficiency | 325 | 5 | 1.9 | |
| Vitamin D Deficiency | 4508 | 11 | 4.2 | |
| Vitamin K Deficiency | 10 | 1 | < 1.0 | |
| Potassium Deficiency | 329 | 7 | 2.7 | |
| Iron Deficiency | 581 | 15 | 5.8 | |
| Magnesium Deficiency | 68 | 4 | 1.5 | |
| Calcium Deficiency | 96 | 7 | 2.7 | |
| Dehydration | 379 | 35 | 13.5 | |
| Malnutrition | 713 | 49 | 18.8 | |
| Daily Activity | Sleep Disorder | 6 | 2 | < 1.0 |
| Substance Abuse | Tobacco Smoking | 159 | 55 | 21.2 |
| Excess Alcohol | 2884 | 25 | 9.6 |
3.4.1. Individual effect
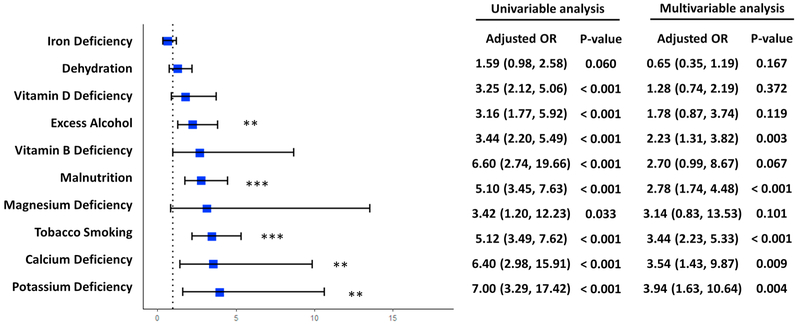
We analyzed the individual effects of the 10 lifestyle exposures that occurred the most frequently in the clinical notes, which were the same between the AD dementia and control groups. Fig. 4 provides the results from both univariable analysis and multivariable analysis. In univariable analysis, after adjustment for age and sex, all 10 exposures were significantly associated with AD dementia, except iron deficiency (adjusted OR: 1.59, 95% CI: (0.98, 2.58)). In the multivariable analysis, after adjustments for age, sex, and each of the other 9 exposures, 5 out of the 10 factors were significantly associated with AD dementia. Potassium deficiency showed the strongest correlation with AD (adjusted OR: 3.94, 95% CI: (1.63, 10.64)) followed by calcium deficiency (adjusted OR = 3.54, 95% CI: (1.43, 9.87)) and tobacco smoking (adjusted OR = 3.44, 95% CI: (2.23, 5.33)). Malnutrition (adjusted OR = 2.78, 95% CI: (1.74, 4.48)) and excess alcohol use (adjusted OR = 2.23, 95% CI: (1.31, 3.82)) were also associated with AD dementia, but the associations were weaker than the aforementioned factors. We did not identify any associations between the AD dementia and the other 5 exposures, including iron deficiency (adjusted OR: 0.65, 95% CI: (0.35, 1.19)), dehydration (adjusted OR: 1.28, 95% CI: (0.74, 2.19)), vitamin D deficiency (adjusted OR: 1.78, 95% CI: (0.87, 3.74)), vitamin B deficiency (adjusted OR: 2.70, 95% CI: (0.99, 8.67)) and magnesium deficiency (adjusted OR: 3.14, 95% CI: (0.83, 13.53)). The effects of lifestyle exposures outside of the top 10 factors (e.g., high fat diet) were not analyzed due to their low frequencies (number of patient ≤ 1 in each group).
Fig. 4.
Odds ratios of top 10 lifestyle exposures identified in clinical notes. Odds ratio of each lifestyle exposure was calculated with adjustment for age and other 9 exposures. * p-value < 0.05; ** p-value < 0.01; *** p-value < 0.001.
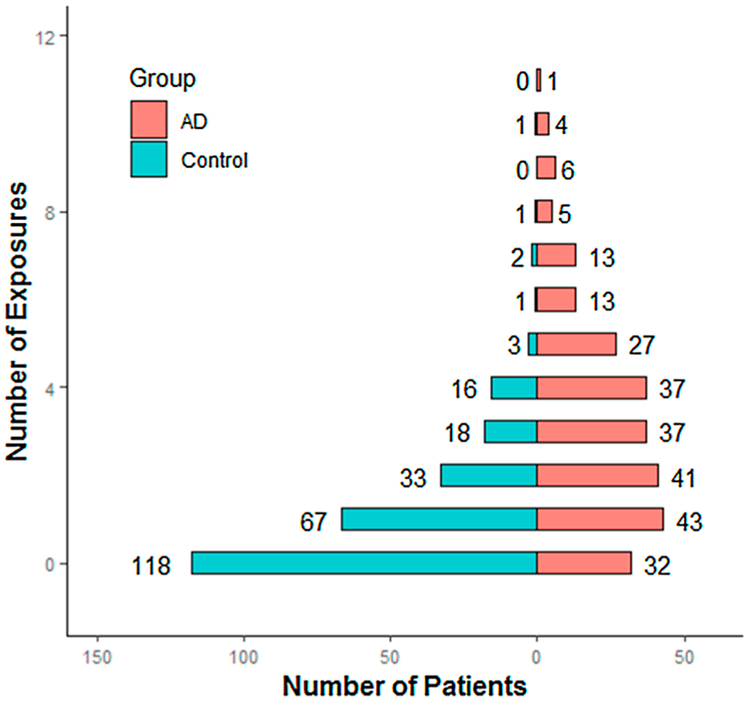
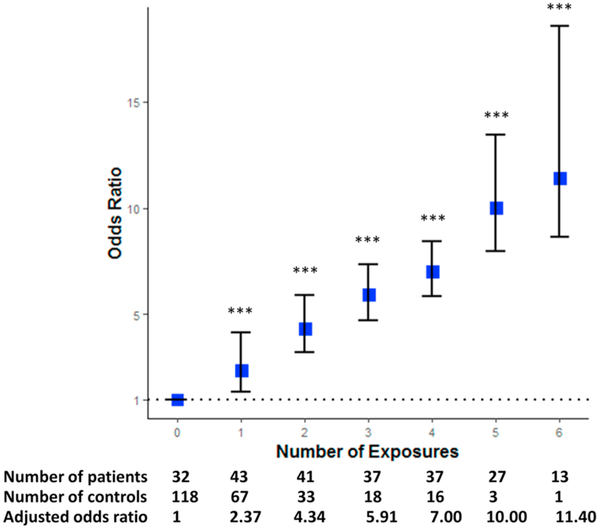
Summarizing all lifestyle exposures, we observed significantly more risk factors in the AD dementia group compared to the control group (χ2 = 120.31, p-value < 0.001). The median of the total number of exposures each patient had was 3 (interquartile range: 1–5) in the AD group whereas the number was 1 (interquartile range: 0–2) in the control group. The distribution of lifestyle exposures between two groups is compared in Fig. 5. Note that we excluded an outlier patient in the AD group who had as much as 20 exposures in total. Fig. 6 shows the odds of AD according to the number of lifestyle exposures. A strong and graded relation was noted between the number of exposures and the presence of AD, with an odds ratio of 13.2 for top (number of exposures = 6) versus the lowest decile of exposure numbers (number of exposures = 0).
Fig. 5.
Distribution of lifestyle exposures between AD group and control group.
Fig. 6.
Odds of AD according to number of lifestyle exposures. *** p-value < 0.001.
3.5. Missing lifestyle exposures
25 out of 55 potential lifestyle exposures identified by literature review were not identified in our dataset, 22 of which were dietary factors (Table 6). 13 dietary factors could not be mapped to any UMLS concept, including dietary excesses (diabetogenic diet, high advanced glycation end products diet, high arachidonic acid, and high unfermented soy), nutrient deficiencies (linoleic acid deficiency, early life nutrient restriction, glutathione depletion, low cocoa, low coffee, low flavonols and low fruit), and food additives (menadione. diacetyl). The other 9 factors could be mapped to UMLS concepts but were not mentioned in clinical notes, including dietary heavy metal intake (high copper diet, high zinc diet), food additives (monosodium glutamate, cysteine), nutrient deficiencies (selenium deficiency, glucose deprivation, low tryptophan diet), high methionine diet and industrialized/preserved food. 3 substance abuses were not found in our patient cohorts, including amphetamine, MDMA, and cocaine/opiates. A full list of the 55 lifestyle exposures not found in our dataset is provided in Table S1 of the supplementary material.
Table 6.
Lifestyle Exposures not found in Clinical Notes.
| Category | Without UMLS concepts | With UMLS concepts |
|---|---|---|
| Dietary Factor | Diabetogenic diet | High copper diet |
| High advanced glycation end products diet | High zinc diet | |
| High arachidonic acid | High methionine diet | |
| High unfermented soy | Low tryptophan diet | |
| Linoleic acid deficiency | Glucose deprivation | |
| Early life nutrient restriction | Selenium deficiency | |
| Glutathione depletion | Industrialized/preserved food | |
| Low cocoa | Monosodium glutamate | |
| Low coffee | Cysteine | |
| Low flavonols | ||
| Low fruit | ||
| Menadione | ||
| Diacetyl | ||
| Substance Abuse | Amphetamine | |
| MDMA | ||
| Cocaine/opiates |
3.6. Performance of the NLP method in MetaMap
The performance of the automated NLP algorithm was compared with the manual review of the random 50 sample documents. Specifically, 73 medical concepts related to risk exposures and interventions were found by MetaMap in the 50 sampled documents, among which the true positive is 54 and the false positive is 19. Thus, the precision of MetaMap is 74.0% based on our limited manual evaluation. Detailed information about the results in terms of frequency, number of true positives, and PPV of each extracted concept and a few typical false positive examples are provided in Tables S2 and S3 of the supplementary material, respectively.
4. Discussion
This is, to the best of our knowledge, the first study that examined the published lifestyle exposures and corresponding intervention strategies for AD patients in routine practice using NLP techniques. By investigating 55 lifestyle exposure factors from 520 clinical notes comprised of 64,672 medical documents, we found evidence of a positive graded relation between AD dementia and the number of lifestyle exposures the patients suffered. The results in this exploratory study show that NLP techniques are able to access much more lifestyle information than could be obtained in commonly used questionnaire assessments for this task. The ability to evaluate large numbers of potential risk factors illustrates the feasibility of NLP techniques for lifestyle risk factor evaluation in a large-scale cross-sectional study.
The 55 lifestyle risk factors investigated in this study were retrieved from different levels of AD-related studies such as neuronal cell culture [32], transgenic animal models [33], brain imaging studies [34], etc. However, only a few of them had been proven to significantly increase the risk of AD in well-designed longitudinal clinical studies. In our dataset, 5 lifestyle exposures were significantly related to the presence of AD, including potassium deficiency, calcium deficiency, tobacco smoking, malnutrition and excessive alcohol use. However, a causal relation between these factors and the onset of AD could not be determined in this cross-sectional study. Large-scale clinical trials should be conducted in the future to further investigate their roles in AD progression. 22 dietary factors were not mentioned in our clinical notes datasets. It is beyond the scope of this work to consider whether all putative risk factors ought to be reported in clinic notes, or used for disease monitoring.
Most current human studies of lifestyle exposures of AD focus on cardiovascular exposures, such as high fat diet [35], high calorie diet [36], and high carbohydrate diet [37]. These factors attract much attention due to their high correlations with metabolic and cardiovascular diseases including diabetes, hypertension, stroke, etc. However, we found that cardiovascular exposures were rarely observed in the clinical notes of the AD cohort. For example, high calorie diet and high meat diet occurred in the clinical notes of only 1 and 2 patients out of 260 patients, respectively. In contrast, nutrient deficiency exposures occurred much more commonly occurred in AD patients’ clinical notes. Over 25 patients were exposed to each of the following: 1) vitamin B deficiency; 2) vitamin D deficiency; 3) potassium deficiency; 4) iron deficiency; 5) calcium deficiency and 6) malnutrition. Vitamin E deficiency, magnesium deficiency and starvation were also found in the clinical notes of our patient cohort. Our findings are in line with previous studies reporting vitamin and mineral insufficiency in patients with AD [38-41]. These studies have investigated the risk or the prevalence of certain nutrient deficiency in AD patients in specific contexts such as in elder AD patients, in elder women, or in the general population. Our study adds to previous findings by reporting the prevalence of nutrient deficiencies 1) using NLP methods to perform complete nutrient assessments, and 2) in other patient groups.
Nutrient supplements were widely identified in our clinical note datasets where we found 14 nutrient supplements, covering vitamins, minerals, vegetables, docosahexaenoic acids and fatty acids. However, the laboratory test results of the circulating concentrations of nutrients were rarely identified in our dataset. For example, among 21 AD patients who took vitamin D supplements in our cohort, only 1 of them had the vitamin D measurements in his medical records. Whether the other 20 patients had satisfactory circulating levels of vitamin D after receiving vitamin D therapy was not mentioned in their clinical notes. In addition, previous studies suggested a remarkably low adherence of nutrition supplements. Miller et al [42] reported a poor adherence (67%) with nutrition supplement prescriptions over 42 days. Modi et al [43] reported a lower adherence (30%) to prescribed multivitamin therapy in patients over six months. Hayes et al [44] reported that the medication adherence was significantly lower among subjects over 65 with cognitive impairments. Finally, some nutrients (e.g., selenium deficiency) were never routinely measured in our dataset. Thus, the intervention strategies mentioned in clinical notes may not cover all nutrient components.
Most previous lifestyle studies of AD focused on a limited number of lifestyle exposures in each survey, thus the synergistic effects or the overall effects of multiple lifestyle habits could not be analyzed. By comparing the distributions of lifestyle exposures between the clinical notes from the AD cohort and those from the control cohort, we found that the clinical notes from the AD group had recorded significantly more exposures than those from the control. This finding confirms the results about previously-studied lifestyle exposures. A positive graded relation was observed between AD dementia and the number of exposures the patient had, without either a threshold or a plateau. In particular, even a single lifestyle exposure is significantly related to AD, suggested the importance of complete lifestyle assessment and modification. AD and many other health consequences might share common lifestyle risk factors with overlapping pathological processes. Martins et al [45] found that abnormal glycolipid metabolism are indicated as central in the pathogenesis of both diabetes and AD. Wells et al [46] demonstrated that nutritional deficiencies in elder patients were strongly associated with cognitive impairment and vascular diseases. Hence monitoring a single risk factor may lead to many health benefits.
MetaMap for extracting information from clinical notes has been evaluated in the previous studies and shown high precision and recall [47]. However, the irregular expressions, abbreviations or misspellings contained in free-text clinical notes might result in misrecognitions. For example, the intervention strategy of vitamin D supplement could not be retrieved from the sentence “She is on calcium carbonate and D”. In addition, MetaMap could not distinguish lifestyle risk factors and interventions. High fat diet could be considered as 1) an unhealthy diet habit, and 2) a special diet regimen suitable for patients who need such nutrition, though the second situation was very rare. Finally, although most negative context could be successfully mapped to negative UMLS terms, some of them might cause false positive results. For example, both “fat free” and “non-fat diet” could be successfully mapped to negative UMLS terms while “not high in fat” was falsely mapped to UMLS Term C0425441- Diet high in saturated fats. Based on the limited evaluation on the random samples, the average accuracy of MetaMap in identifying UMLS concepts associated with lifestyle exposures and interventions is 74.0%.
There are several limitations in our study that need to be considered when interpreting the results. First, we did not develop comprehensive algorithms to map UMLS concepts and account for lifestyle exposure terms that could not be mapped to UMLS concepts. Several dietary exposures with complex expressions could not be directly mapped to any UMLS concepts, such as early life nutrient restriction and high advanced glycation end products diet. A keywords-based search could be an alternative approach for investigation of these exposures in a future study. This research was a pilot study to investigate the potential of using NLP algorithm to extract lifestyle exposures from clinical notes for clinical research. In future work, we would like to develop a more comprehensive algorithm that could identify the medical concepts that are related but linguistically different, and improve the NLP method using more heuristic rules or leveraging machine learning approaches to find lifestyle exposures that could not be mapped to UMLS concepts. Second, we did not have readily available data on patients’ education level from EHRs. Therefore, the association between the presence of AD and exposure factors may reflect disturbances associated with socioeconomic or education level. Third, the length of medical records varies among patients. For patients who lived in Rochester, MN for a short time and only had records for a few visits, lifestyle information may not be completely recorded in their clinical documents. The results may also be biased due to the difference between patients and controls in the number of visits. The average number of visits for each patient in the case group is 214 while this number is 35 for the control group. During the control selection, we did not match the number of visits or the number of notes between the case and control. The reason is that the lifestyle information is usually recorded as part of clinical notes during any patient’s visit. Having said that, the patients with more visits may possibly generate more information about lifestyle exposures than those in the control, which may introduce bias to this study. Finally, we cannot confirm that these lifestyle exposures are risk factors leading to AD since we included all clinical notes of patients, involving visits before and after AD diagnosis for studying both risk factors and interventions. It is also possible that AD leads to these lifestyle exposures, such as potassium deficiency and calcium deficiency. In future work, we will extract risk factors and intervention information from notes of visits before and after AD diagnosis, respectively.
5. Conclusion
The results in this exploratory study illustrate the feasibility of NLP techniques for evaluating multiple lifestyle risk factors in a large-scale cross-sectional study by using EHRs. In contrast to contemporary questionnaire-based lifestyle investigations which missed the risk factors that are outside the scope of research interests, our novel NLP approach allows a complete lifestyle assessment in an efficient and costeffective manner. With an accurate automatic method for investigating lifestyle exposures, larger-scale epidemiologic research could be feasibly conducted.
Supplementary Material
Acknowledgements
This work was supported by NIH grants UL1TR02377 Supplement, U01TR002062, and R01LM11934. The funders had no role in the design of the study, or collection, analysis, and interpretation of data and in preparation of the manuscript. The views presented in this report are not necessarily representative of the funder’s views and belong solely to the authors.
Footnotes
Declaration of Competing Interest
None.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ijmedinf.2019.08.003.
References
- [1].Yankner BA, Mechanisms of neuronal degeneration in Alzheimer’s disease, Neuron 16 (5) (1996) 921–932. [DOI] [PubMed] [Google Scholar]
- [2].Association, A.s, Alzheimer’s Disease Facts and Figures, Available from: (2018) https://www.alz.org/alzheimers-dementia/facts-figures.
- [3].Arab L, Sabbagh MN, Are certain lifestyle habits associated with lower Alzheimer’s disease risk? J. Alzheimers Dis. 20 (3) (2010) 785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].van Duijn CM, Epidemiology of the dementias: recent developments and new approaches, J. Neurol. Neurosurg. Psychiatry 60 (5) (1996) 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Launer LJ, et al. , Rates and risk factors for dementia and Alzheimer’s disease: results from EURODEM pooled analyses. EURODEM Incidence Research Group and Work Groups. European Studies of Dementia, Neurology 52 (1) (1999) 78–84. [DOI] [PubMed] [Google Scholar]
- [6].Sempos CT, Invited commentary - some limitations of semiquantitative food frequency questionnaires, Am. J. Epidemiol. 135 (10) (1992) 1127–1132. [Google Scholar]
- [7].Berti V, et al. , Nutrient patterns and brain biomarkers of Alzheimer’s disease in cognitively normal individuals, J. Nutr. Health Aging 19 (4) (2015) 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gardener S, et al. , Adherence to a Mediterranean diet and Alzheimer’s disease risk in an Australian population, Transl. Psychiatry 2 (2012) el64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gauthier E, et al. , Environmental pesticide exposure as a risk factor for Alzheimer’s disease: a case-control study, Environ. Res. 86 (1) (2001) 37–45. [DOI] [PubMed] [Google Scholar]
- [10].Mosconi L, et al. , Lifestyle and vascular risk effects on MRI-based biomarkers of Alzheimer’s disease: a cross-sectional study of middle-aged adults from the broader New York City area, BMJ Open 8 (3) (2018) p. e019362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rogers MA, Simon DG, A preliminary study of dietary aluminium intake and risk of Alzheimer’s disease, Age Ageing 28 (2) (1999) 205–209. [DOI] [PubMed] [Google Scholar]
- [12].Kim EH, et al. , Challenges to using an electronic personal health record by a lowincome elderly population, J. Med. Internet Res. 11 (4) (2009) e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang Y, et al. , Clinical information extraction applications: a literature review, J. Biomed. Inform. 77 (2018) 34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kipper-Schuler Karin, James Masanz VK, Ogren Philip, Savova Guergana, System evaluation on a named entity corpus from clinical notes, Language Resources and Evaluation Conference, LREC, (2008). [Google Scholar]
- [15].Meng F, et al. , Automatic generation of repeated patient information for tailoring clinical notes, Int. J. Med. Inform. 74 (7–8) (2005) 663–673. [DOI] [PubMed] [Google Scholar]
- [16].Cao H, Stetson P, Hripcsak G, Assessing explicit error reporting in the narrative electronic medical record using keyword searching, J. Biomed. Inform. 36 (1–2) (2003) 99–105. [DOI] [PubMed] [Google Scholar]
- [17].Hripcsak G, et al. , Use of natural language processing to translate clinical information from a database of 889,921 chest radiographic reports, Radiology 224 (1) (2002) 157–163. [DOI] [PubMed] [Google Scholar]
- [18].Cambria E, White B, Jumping NLP curves: a review of natural language processing research, IEEE Comput. Intell. Mag. 9 (2) (2014) 48–57. [Google Scholar]
- [19].Mehrotra A, et al. , Applying a natural language processing tool to electronic health records to assess performance on colonoscopy quality measures, Gastrointest. Endosc. 75 (6) (2012) p. 1233–9 el4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kaur H, et al. , Automated chart review utilizing natural language processing algorithm for asthma predictive index, BMC Pulm. Med. 18 (1) (2018) 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhong QY, et al. , Screening pregnant women for suicidal behavior in electronic medical records: diagnostic codes vs. clinical notes processed by natural language processing, BMC Med. Inform. Decis. Mak. 18 (1) (2018) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].DeJesus RS, Use of a clinical decision support system to increase osteoporosis screening, J. Eval. Clin. Pract. 18 (4) (2012) 926. [DOI] [PubMed] [Google Scholar]
- [23].Pratt W, Yetisgen-Yildiz M, A study of biomedical concept identification: MetaMap vs. people, AMI A Annu Symp Proc, (2003), pp. 529–533. [PMC free article] [PubMed] [Google Scholar]
- [24].Aronson AR, Lang FM, An overview of MetaMap: historical perspective and recent advances, J. Am. Med. Inform. Assoc. 17 (3) (2010) 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bodenreider O, The unified medical language system (UMLS): integrating biomedical terminology, Nucleic Acids Res. 32 (2004) D267–70 (Database issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Meystre S, Haug PJ, Evaluation of medical problem extraction from electronic clinical documents using MetaMap transfer (MMTx), Stud. Health Technol. Inform. 116 (2005) 823–828. [PubMed] [Google Scholar]
- [27].Shah NH, et al. , Comparison of concept recognizers for building the Open Biomedical Annotator, BMC Bioinformatics 10 Suppl 9 (2009) S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Reátegui R, Ratté S, Comparison of MetaMap and cTAKES for entity extraction in clinical notes, BMC Med. Inform. Decis. Mak. 18 (3) (2018) 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wu Y, et al. , A comparative study of current clinical natural language processing systems on handling abbreviations in discharge summaries, AMIA Annual Symposium Proceedings (2012). [PMC free article] [PubMed] [Google Scholar]
- [30].Kostoff RN, Porter AL, Buchtel HA, Prevention and Reversal of Alzheimer’s Disease: Treatment Protocol, (2018). [Google Scholar]
- [31].Monti JM, Sleep laboratory and clinical-studies of the effects of triazolam, flunitrazepam and flurazepam in insomniac patients, Methods Find. Exp. Clin. Pharmacol. 3 (5) (1981) 303–326. [PubMed] [Google Scholar]
- [32].Kawahara M, et al. , Alzheimer’s beta-amyloid, human islet amylin, and prion protein fragment evoke intracellular free calcium elevations by a common mechanism in a hypothalamic GnRH neuronal cell line, J. Biol. Chem. 275 (19) (2000) 14077–14083. [DOI] [PubMed] [Google Scholar]
- [33].Sensi SL, et al. , Altered oxidant-mediated intraneuronal zinc mobilization in a triple transgenic mouse model of Alzheimer’s disease, Exp. Gerontol. 43 (5) (2008) 488–492. [DOI] [PubMed] [Google Scholar]
- [34].Saar G, et al. , Laminar specific detection of APP induced neurodegeneration and recovery using MEMRI in an olfactory based Alzheimer’s disease mouse model, Neuroimage 118 (2015) 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Laitinen MH, et al. , Fat intake at midlife and risk of dementia and Alzheimer’s disease: a population-based study, Dement. Geriatr. Cogn. Disord. 22 (1) (2006) 99–107. [DOI] [PubMed] [Google Scholar]
- [36].Luchsinger JA, et al. , Caloric intake and the risk of Alzheimer disease, Arch. Neurol. 59 (8) (2002) 1258–1263. [DOI] [PubMed] [Google Scholar]
- [37].Young KW, et al. , A randomized, crossover trial of high-carbohydrate foods in nursing home residents with Alzheimer’s disease: associations among intervention response, body mass index, and behavioral and cognitive function, J. Gerontol. A Biol. Sci. Med. Sci. 60 (8) (2005) 1039–1045. [DOI] [PubMed] [Google Scholar]
- [38].Brewer GJ, et al. , Subclinical zinc deficiency in Alzheimer’s disease and Parkinson’s disease, Am. J. Alzheimers Dis. Other Demen. 25 (7) (2010) 572–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Clarke R, et al. , Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease, Arch. Neurol. 55 (11) (1998) 1449–1455. [DOI] [PubMed] [Google Scholar]
- [40].Littlejohns TJ, et al. , Vitamin D and the risk of dementia and Alzheimer disease, Neurology 83 (10) (2014) 920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sato Y, Asoh T, Oizumi K, High prevalence of vitamin D deficiency and reduced bone mass in elderly women with Alzheimer’s disease, Bone 23 (6) (1998) 555–557. [DOI] [PubMed] [Google Scholar]
- [42].Miller MD, et al. , Adherence to nutrition supplements among patients with a fallrelated lower limb fracture, Nutr. Clin. Pract. 20 (5) (2005) 569–578. [DOI] [PubMed] [Google Scholar]
- [43].Modi AC, et al. , Adherence to vitamin supplementation following adolescent bariatric surgery, Obesity (Silver Spring) 21 (3) (2013) E190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hayes TL, et al. , Medication adherence in healthy elders: small cognitive changes make a big difference, J. Aging Health 21 (4) (2009) 567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Martins I, et al. , Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer’s disease and cardiovascular disease, Mol. Psychiatry 11 (8) (2006) 721. [DOI] [PubMed] [Google Scholar]
- [46].Wells JL, Dumbrell AC, Nutrition and aging: assessment and treatment of compromised nutritional status in frail elderly patients, Clin. Interv. Aging 1 (1) (2006) 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Reategui R, Ratte S, Comparison of MetaMap and cTAKES for entity extraction in clinical notes, BMC Med. Inform. Decis. Mak. 18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.