Abstract
OBJECTIVES
The aim of our study was to investigate the effects of folic acid on cisplatin-induced ototoxicity.
MATERIALS and METHODS
Thirty Wistar albino rats were divided into five groups. Group I received intraperitoneal cisplatin (IP) 10 mg/kg/day and IP folic acid 10 mg/kg/day; Group II received IP cisplatin 10 mg/kg/day and IP physiological saline; Group III received IP cisplatin 10 mg/kg/day and intratympanic (IT) folic acid 0.15 mL/day; Group IV received IP cisplatin 10 mg/kg/day and IT physiological saline; and Group V received IT folic acid 0.15 mL/day. Before and after drug administration, plasma homocysteine, folic acid levels, and auditory brainstem evoked responses (ABR) were measured. The rats were then sacrificed, and the inner ears were processed for electron microscopy.
RESULTS
The differences of ABR thresholds in Group I compared to Group II were significantly smaller at 4 kHz, 8 kHz, and 16 kHz, whereas they were smaller but not statistically significant at 12 kHz in ABR. The differences of ABR thresholds in Group III compared to Group IV were significantly smaller at 12 kHz, and smaller but not statistically significant at 4 kHz, 8 kHz, and 16 kHz. Cisplatin treatment resulted in the degeneration of the cells of the organ of Corti, stria vascularis, and spiral ganglion. The cells of the organ of Corti, stria vascularis, and spiral ganglion showed a partially preserved morphology in both Group I and Group III.
CONCLUSION
Our study results suggests that folic acid is a potential agent in preventing cisplatin-induced ototoxicity.
Keywords: Cisplatin, folic acid, ototoxicity, hearing loss, electron microscope
INTRODUCTION
Ototoxicity is defined as functional impairment of the inner ear caused by a drug or a chemical substance. This functional impairment can manifest as hearing loss, imbalance, or both. Common drugs causing ototoxicity include antibiotics, anti-neoplastic drugs, diuretics, chelating agents, anti-inflammatory drugs, anti-malarial drugs, and topical agents [1–4].
Cisplatin is an effective anti-neoplastic agent possessing ototoxic effects. Cisplatin-induced ototoxicity has become the most common dose-limiting side effect due to the fact that it is more commonly observed, and more patients are affected when it is compared to other side effects [5].
Due to the absence of an ideal otoprotective agent for clinical use, a safe and effective agent protecting against cisplatin-induced ototoxicity is required to increase the quality of life of patients and prevent dose-limiting side effects of cisplatin therapy [6, 7].
Folic acid and its active form have been found to possess direct and indirect antioxidant activity [8]. Considering the fact that folic acid is a neuroprotective and antioxidant agent [9–11] and the studies reporting that low serum folic acid levels could be associated with hearing impairment,[12–14] in the present study, we aimed to investigate the use of folic acid as a protective agent in an experimental rat model of cisplatin-induced ototoxicity, to evaluate the protective effects of folic acid using auditory brainstem responses (ABR), and to compare plasma folic acid and homocysteine levels before and after folic acid administration.
MATERIALS AND METHODS
This study was approved by the local ethical committee (Date: 04/06/2015; number: 370). The study was conducted in accordance with the guidelines of the Helsinki Declaration relevant to experimental studies. In addition, international standards regarding the animal care and handling have been followed during the experiments. All the animals were transported to the local experimental animal research laboratory and kept in cages under standard conditions and fed with standard nutrition. Official experimental certificates were provided for each animal used in the study.
Animals
Male Wistar albino rats weighting 250–300 gr were used in this study. All rats were housed at a temperature of 24±3°C with a 12-hour light-dark cycle and acclimated for 7 days before the study. The animals were fed with a standard pellet diet and water ad libitum.
Drug Preparation and Administration
Cisplatin (Cisplatin; Hospira UK Ltd., Maidenhead, UK) and folic acid (Leucovorin-Teva; Teva Med-Pharmaceuticals Industry and Trade Inc., Tel-Aviv, Israel) were used.
A total of 30 Wistar albino male rats were divided into five groups, six animals in each (Table 1). Intraperitoneal cisplatin (CDDP) (10 mg/kg/day) was administrated on Days 2 and 3 to all groups except Group V. Besides CDDP, Group I was administrated intraperitoneally (IP) 10 mg/kg/day folic acid (FA); Group II was administrated IP saline; Group III was administrated intratympanic (IT) 0.15 mL/day FA; Group IV was administrated IT saline on Days 1, 2, 3, and 4; Group V was administrated only IT 0.15 mL/day FA for 4 days.
Table 1.
Experimental groups in the study
| Group | Procedure | Number of Rats |
|---|---|---|
| Group I | Intraperitoneal cisplatin+intraperitoneal folic acid (IP CDDP+IP FA) | 6 |
| Group II | Intraperitoneal cisplatin+intraperitoneal physiological saline (IP CDDP+IP saline) | 6 |
| Group III | Intraperitoneal cisplatin+intratympanic folic acid (IP CDDP+IT FA) | 6 |
| Group IV | Intraperitoneal cisplatin+intratympanic physiological saline (IP CDDP+IT saline) | 6 |
| Group V | Intratympanic folic acid (IT FA) | 6 |
IP: intraperitoneal; CDDP: cisplatin; FA: folic acid; IT: intratympanic
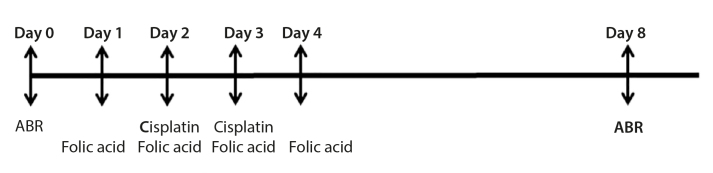
Baseline hearing ABR measurements before drug administration were obtained in all rats. Considering the start day as Day 1, Group I received IP FA (10 mg/kg/day) on Days 1, 2, 3 and 4, and IP CDDP (10 mg/kg/day) on Days 2 and 3 with a cumulative dose of 20 mg/kg. Group II received IP physiological saline (2 mL/day) on Days 1, 2, 3, and 4 and IP CDDP (10 mg/kg/day) on Days 2 and 3 with a cumulative dose of 20 mg/kg. The rats in Group III underwent myringotomy to the anterosuperior quadrant of the tympanic membrane using a dental injector (28 Gauge) and received IT FA (0.15 mL/day) on Days 1, 2, 3, and 4 and IP CDDP (10 mg/lg/day) on Days 2 and 3 with a cumulative dose of 20 mg/kg. Group IV received IT physiological saline on Days 1, 2, 3, and 4 and IP CDDP (10 mg/kg/day) on Days 2 and 3 with a cumulative dose of 20 mg/kg. Group V received IT FA (0.15 mL/day) on Days 1, 2, 3, and 4. The study plan is presented in Figure 1.
Figure 1.
Study plan. Baseline hearing ABR measurements were obtained on Day 0 before drug administration in all rats. Group I received IP FA (10 mg/kg/day) on Days 1, 2, 3, and 4, and IP CDDP (10 mg/kg/day) on Days 2 and 3, with a cumulative dose of 20 mg/kg. Group II received IP physiological saline (2 mL/day) on Days 1, 2, 3, and 4 and IP CDDP (10 mg/kg/day) on Days 2 and 3, with a cumulative dose of 20 mg/kg. The rats in Group III received IT FA (0.15 mL/day) on Days 1, 2, 3, and 4 and IP CDDP (10 mg/lg/day) on Days 2 and 3. Group IV received IT physiological saline on Days 1, 2, 3, and 4 and IP CDDP (10 mg/kg/day) on Days 2 and 3. Group V received IT FA (0.15 mL/day) on Days 1, 2, 3, and 4. After drug administration, ABR measurements were obtained on Day 8. Biochemical measurements were done on Day 0 (pre) and Day 8 (post). The animals were sacrificed on Day 8 for an ultrastructural examination of the inner ear.
Measurement of Plasma Homocysteine and Folic Acid Levels
The rats in Groups I, II, and III were anesthetized using a combination of ketamine hydrochloride 75 mg/kg (Ketalar; Eczacibasi, Istanbul, Turkey) and xylazine 7.5 mg/kg (Rompun; Bayer, Leverkusen, Germany) given IP, and blood was drawn from the lateral tail veins to determine baseline plasma folic acid and homocysteine levels. The samples were processed using auto analyzers Architect (Architect i2000SR; Abbott Diagnostics, Abbott Park, IL, USA) and Immulite (Immulite 2000; Siemens, Erlangen, Germany) devices. The rats were anesthetized in the same fashion after drug injections and the evaluation of ABR scores after the procedure, and blood samples were collected for the measurement of plasma folic acid and homocysteine levels.
Auditory Evoked Potentials and Recording
All rats used in the study were anesthetized by the combination of IP ketamine hydrochloride 75 mg/kg (Ketalar, Eczacibasi, Istanbul, Turkey) and xylazine 7.5 mg/kg (Rompun, Bayer, Leverkusen, Germany). Rats were placed on a warm sheet. Using an otomicroscope (Takagi Operating Microscopr, Mfg. Co. Ltd, Japan), an appropriate size ear speculum was inserted into the external auditory canal of the rats, and tympanic membranes were examined. ABR measurements were performed in the right ear of the anesthetized rats using a Neurosoft Neuro-audio (Neurosoft Neuro-audio; Neurosoft Ltd. Ivanova, Russia) device. Stainless steel 20x0.30 mm needle electrodes were used, and the ground electrode was placed at the glabella, the reference electrode was placed subcutaneously at the right and left mastoid area, and active electrode was placed subcutaneously at the vertex. The impedance was kept at 0 Kohm when the electrodes touched each other and at 5 Kohm after the placement on the rats. In ABR measurements, stimulation velocity was 13.00 rate, the analysis time was 10 msec, and the averaging was 1000 sweep. The recording was started at 70 dB nHL, and the intensity was changed depending on the responses. A tone-burst stimulation (0.2 rise-fall time and 1 msec duration) at 4, 8, 12, and 16 kHz was used for frequency-specific threshold estimates. The threshold was defined as the lowest volume intensity that is visually detectable and reproducible. For ABR measurements 8 days after the baseline measurements (4 days after the last cisplatin administration), the rats were anesthetized, and their ears were examined under the microscope to rule out external and middle ear infections. Hearing thresholds of rats in all groups were determined using ABR measurements, and the results were compared with baseline ABR measurements obtained before drug administration.
Histopathological Study
For an ultrastructural investigation, temporal bones of Groups I, II, III, and V were carefully harvested, soft tissues were removed, and cochleas were fixed in a 2.5% glutaraldehyde solution in a phosphate buffer, pH 7.4, for 4 h. The specimens were decalcified by the immersion in ethylenediaminetetraacetic acid solution on a tissue rotator. When the bony capsule of the cochlea was soft enough, the specimens were placed in gluteraldehyde for 1 hour after washing in the phosphate buffer. Then, they were post-fixed for 1 h in 1% osmium tetroxide in 0.1 M phosphate buffer. After washing in the phosphate buffer, they were dehydrated in a graded series of ethanol to absolute ethanol, treated with propylene oxide, and embedded in Araldite/Epon812 (Araldite/Epon812; EMS, Hatfield, PA, USA). After heat polymerization, sections were cut using a microtome. Semi-thin sections were stained with methylene blue-azure II and examined using a light microscope (Leica Microsystems, Wetzlar, Germany) with a DC490 digital camera (Leica Microsystems, Wetzlar, Germany). Photographs were taken from the basal turn of the cochlea, and samples were evaluated for the tissue alterations such as the degeneration of cells, and edema in the organ of Corti, stria vascularis, and spiral ganglion. These changes were graded as absent (0), mild (1), moderate (2), or severe (3).
Ultrathin sections (Leica ultracut R) were double-stained with uranyl acetate and lead citrate (Leica EM AC20; Leica Microsystems, Wetzlar, Germany). These sections were examined under a JEOL-JEM 1400 electron microscope and photographed by a CCD camera (Gatan Inc., Pleasanton, CA, USA).
Statistical Analysis
The data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 16.0 (SPSS Inc., Chicago, IL, USA). Descriptive data for numeric variables were expressed as the mean, standard deviation, median, minimum, and maximum. The data were tested for their fitness to normal distribution. Quantitative data were compared between the groups using the Kruskal–Wallis test, and post-hoc analyses were used to determine the group showing significant difference. The Wilcoxon signed-rank test was used to make an intragroup comparison. A p<0.05 was considered statistically significant.
RESULTS
Comparison of the Hearing Losses Before and After Drug Administration
When the differences in ABR thresholds before and after drug administration were compared between the groups, there was a significant difference between Group I and Group II at 4 kHz (p=0.030), 8kHz (p=0.004), and 16 khz (p=0.005). IP folic acid caused a significant decrease in the differences of ABR thresholds associated with cisplatin therapy at 4, 8, 16 kHz. There was no statistically significant difference between Group I and Group II at 12 kHz (p=0.113). However, the difference in the ABR thresholds at 12 kHz in Group I was smaller than in Group II. There was no statistically significant difference between Group III and Group IV at 4 kHz (p=0.408), 8 kHz (p=0.166), and 16 kHz (p=0.074), although the difference in the ABR thresholds at 4, 8, and 16 kHz in Group III was lower than in Group IV. There was a significant difference between Group III and Group IV at 12 kHz (p=0.006) (Table 2, Figure 2).
Table 2.
Assessment of hearing loss (frequency-specific threshold shifts in between the groups)
| Group I | Group II | Group III | Group IV | Group V | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| Frequency-specific difference | Median Min Max |
Mean St. D. (dB) | Median Min Max |
Mean St. D. (dB) | Median Min Max |
Mean St. D. (dB) | Median Min Max |
Mean St. D. (dB) | Median Min Max |
Mean St. D. (dB) |
| 4 kHz | 15 | 15.0 | 30 | 28.3 | 15 | 13.3 | 30 | 25.0 | 0 | −1.6 |
|
| ||||||||||
| 10 | 5.4 | 20 | 4.0 | −10 | 13.6 | 10 | 0 | −10 | 4.0 | |
|
| ||||||||||
| 20 | 30 | 30 | 30 | 8.3 | 0 | |||||
|
| ||||||||||
| 8 kHz | 10 | 11.6 | 30 | 30.0 | 15 | 13.3 | 30 | 28.3 | 0 | 0.0 |
|
| ||||||||||
| 10 | 4.0 | 30 | 0.0 | −10 | 13.6 | 20 | 7.5 | −10 | 6.3 | |
|
| ||||||||||
| 20 | 30 | 30 | 40 | 10 | ||||||
|
| ||||||||||
| 12 kHz | 20 | 18.3 | 40 | 43.3 | 20 | 15.0 | 50 | 48.3 | 0 | 1.6 |
|
| ||||||||||
| 10 | 7.5 | 40 | 5.1 | 0 | 8.3 | 30 | 11.6 | 0 | 4.0 | |
|
| ||||||||||
| 30 | 50 | 20 | 60 | 10 | ||||||
|
| ||||||||||
| 16 kHz | 15 | 13.3 | 50 | 50.0 | 20 | 18.3 | 50 | 45.0 | 0 | 3.3 |
|
| ||||||||||
| −10 | 13.6 | 40 | 6.3 | 0 | 9.8 | 20 | 13.7 | 0 | 5.1 | |
|
| ||||||||||
| 30 | 60 | 30 | 60 | 10 | ||||||
Figure 2.
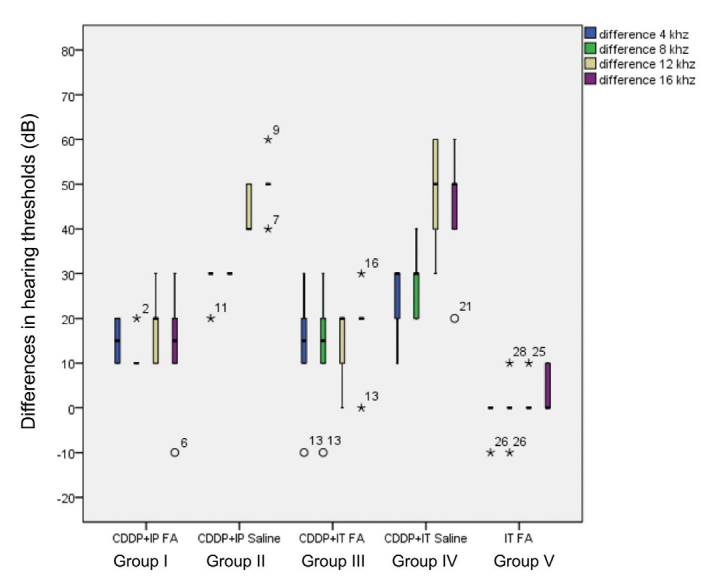
Comparison of differences in hearing thresholds before and after drug administration. A significant difference was detected between Group I and Group II at 4 kHz (p=0.030), 8kHz (p=0.004), and 16 kHz (p=0.005). There was no statistically significant difference between Group I and Group II at 12 kHz (p=0.113). There was a significant difference between Group III and Group IV at 12 kHz (p=0.006), and no statistically significant difference was detected between Group III and Group IV at 4 kHz (p=0.408), 8 kHz (p=0.166), and 16 kHz (p=0.074).
Folic Acid and Homocysteine Levels
When plasma folic acid and homocysteine levels before and after drug administration were evaluated in rats that received IP folic acid and IP cisplatin (Group I), plasma folic acid levels after drug administration were found to be significantly higher (p=0.028). There was a decrease in homocysteine levels after drug administration; however, this decrease was not statistically significant (p=0.075). In Group II, no statistically significant change was found in plasma folic acid levels after drug administration (p=0.498). There was an increase in plasma homocysteine levels after drug administration, and this was found to be statistically significant (p=0.027). In rats that received IP cisplatin and IP physiological saline (Group III), no statistically significant change was found in plasma folic acid levels after drug administration (p=0.498). There was an increase in plasma homocysteine levels after drug administration, and this was found to be statistically significant (p=0.027) (Table 3).
Table 3.
Assessment of folate and homocysteine levels of the groups before and after drug administration
| Group Ia | Group IIa | Group IIIa | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Pre | Post | p | Pre | Post | p | Pre | Post | p | |
| Folate level (ng/mL) | 18.65 (17.10–19.50) | 91.10 (38.70–98.90) | 0.028* | 17.85 (17.40–18.70) | 18.35 (17.20–19.00) | 0.498 | 17.55 (16.90–18.60) | 18.20 (17.40–18.80) | 0.141 |
|
| |||||||||
| Homocystein level (Umol/L) | 2.75 (2.43–3.69) | 2.31 (2.04–3.51) | 0.075 | 2.50 (2.00–4.28) | 2.89 (2.10–4.67) | 0.027* | 3.53 (2.63–4.37) | 3.70 (2.71–4.29) | 0.092 |
Data represent median (min-max) values.
p<0.05.
Light Microscopic Examination
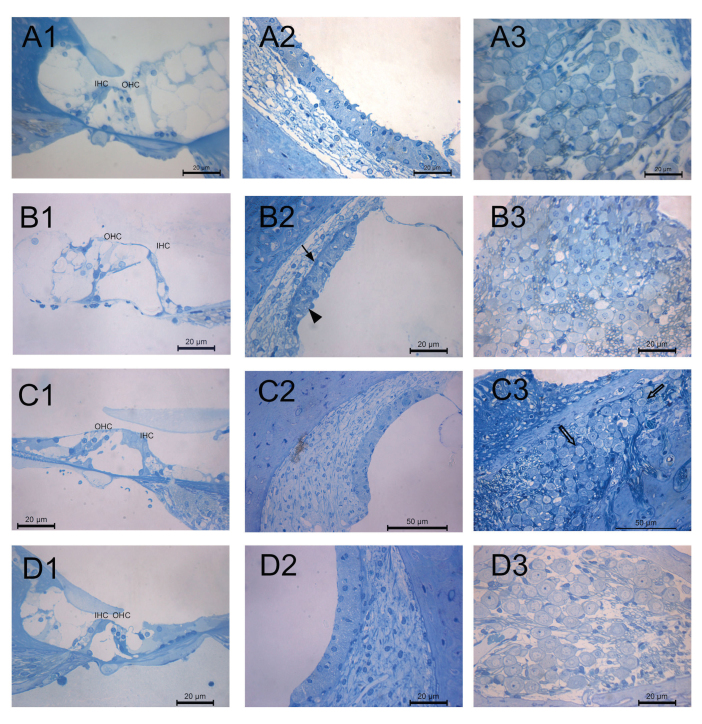
The outer and inner hair cells supported by phalangeal cells were examined in all groups. The nuclei of the outer hair cells were condensed, and vacuoles were present in the inner phalangeal cells in Group I (CDDP+IP FA) (Figure 3a1). Cisplatin treatment resulted in the degeneration of the organ of Corti cells. In the section of organ of Corti, although the apical part of the outer hair cells was in contact with each other, the cytoplasm of the cells was swollen, and outer hair cells were degenerated in Group II (CDDP). There were gaps in the junction of outer hair and outer phalangeal cells (Figure 3b1). The basal parts of the outer hair cells were supported by outer phalangeal cells in Group III (CDDDP+IT FA). There were numerous vacuoles in the cytoplasm of inner phalangeal cells in this group (Figure 3c1) The outer phalangeal cells were surrounding the basal part of the outer hair cells in Group V (IT FA) (Figure 3d1).
Figure 3. a–d.
Representative light micrographs showing 1) the organ of Corti, 2) stria vascularis, and 3) spiral ganglion of cochlea specimens. Degenerated outer hair cells in the organ of Corti, basal cells with condensed nuclei (arrow) and vacuoles (arrow head) in the stria vascularis, and karyorrhexis in the ganglion cells of cisplatin treated group. A few degenerated ganglion cells (empty arrow) in the CDDP+IT FA group. OHC, outer hair cell; IHC, inner hair cell. Group I (CDDP+IP FA) (a); Group II (CDDP) (b); Group III (CDDP+IT FA) (c); Group V (IT FA) (d). Methylene blue-Azure II.
The intermediate cells were sparse in Group I (CDDP+IP FA) (Figure 3a2). There were vacuoles in the cytoplasm of the marginal cells, and basal cells were squamous with condensed nuclei in stria vascularis of Group II (CDDP) (Figure 3b2). Stasis was detected in the capillaries of stria vascularis in Group III (CDDP+IT FA) (Figure 3c2). Normal morphology of stria vascularis was detected in Group V (IT FA) (Figure 3d2).
Ganglion cells with euchromatic nuclei and prominent nucleolus were detected in Group I and Group V. Karyorrhexis was detected in the section of the spiral ganglion of Group II. A few degenerated ganglion cells were detected in the spiral ganglion of Group III (Figure 3). Histopathological scores are presented in Table 4.
Table 4.
Histopathological evaluation of the inner ear in experimental groups
| Group Ia (CCDP + IP FA) n=6 |
Group IIa (CDDP + IP saline) n=6 |
Group IIIa (CCDP + IT FA) n=6 |
Group Va (IT FA) n=6 |
|
|---|---|---|---|---|
| Degeneration in the organ of corti | ||||
| Absent (0) | 0 | 0 | 3 | 5 |
| Mild (1) | 5 | 0 | 3 | 1 |
| Moderate (2) | 1 | 2 | 0 | 0 |
| Severe (3) | 0 | 4 | 0 | 0 |
| Epithelial degeneration in stria vascularis | ||||
| Absent (0) | 4 | 0 | 3 | 6 |
| Mild (1) | 1 | 0 | 3 | 0 |
| Moderate (2) | 1 | 5 | 0 | 0 |
| Severe (3) | 0 | 1 | 0 | 0 |
| Edema in stria vascularis | ||||
| Absent (0) | 6 | 3 | 3 | 5 |
| Mild (1) | 0 | 2 | 3 | 1 |
| Moderate (2) | 0 | 1 | 0 | 0 |
| Severe (3) | 0 | 0 | 0 | 0 |
| Edema in spiral ganglion | ||||
| Absent (0) | 2 | 4 | 6 | 6 |
| Mild (1) | 4 | 1 | 0 | 0 |
| Moderate (2) | 0 | 0 | 0 | 0 |
| Severe (3) | 0 | 1 | 0 | 0 |
| Ganglion cell degeneration in spiral ganglion | ||||
| Absent (0) | 0 | 0 | 0 | 6 |
| Mild (1) | 2 | 1 | 6 | 0 |
| Moderate (2) | 4 | 1 | 0 | 0 |
| Severe (3) | 0 | 4 | 0 | 0 |
Samples of the inner ear were evaluated for the tissue alterations such as the degeneration of cells and edema in the organ of Corti, stria vascularis, and spiral ganglion. These changes were graded as absent (0), mild (1), moderate (2), or severe (3).
CCDP+IP FA: cisplatin and then intraperitoneal folic acid administration; CCDP+IT FA: cisplatin and then intratympanic folic acid administration; IT FA: intratympanic folic acid.
Electron Microscopic Examination
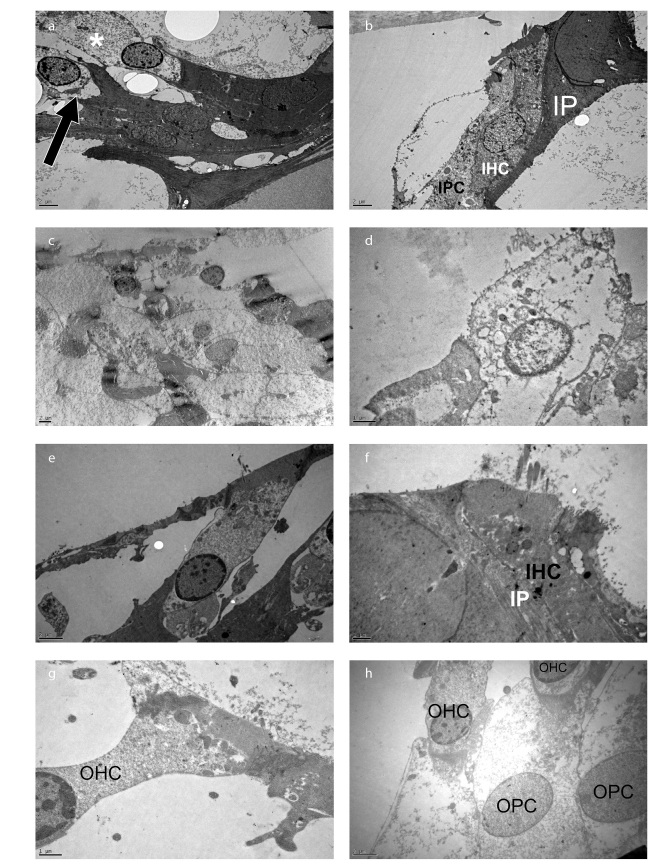
The stereocilia of the outer and inner hair cells were examined in Group I (CDDP+IP FA). Beside the lysosome and mitochondria, degenerative areas were observed in the cytoplasm of the outer hair cells. Degeneration was also seen at the basal part of the outer hair cell where the afferent and efferent nerve fibers were located in this group (Figure 4a). The cell boundaries of the inner hair cell in Group I were regular, and intercellular junctions were normal (Figure 4b). Cisplatin treatment resulted in the degeneration of hair cells with swollen cytoplasm and degenerated organelles (Figures 4c, 4d).
Figure 4. a–h.
Degenerative areas in the cytoplasm (asterisk) and at the basal part (arrow) of the outer hair cells in Group I (CDDP+IP FA). The cell boundaries of inner hair cells were regular, and intercellular junctions were normal in Group I. Degenerated hair and phalangeal cells in the organ of Corti in Group II. Outer and inner hair cells with an intact cell membrane, junctional complexes, and homogenous cytoplasm in Group III (CDDP+IT FA). Outer hair cells with an intact cell membrane in Group V (IT FA). Group I (CCDP+IP FA) (a, b); Group II (CDDP) (c, d); Group III (CCDP+IT FA) (e, f); and Group V (IT FA) (g, h). X6000 (a, b), X4000 (c), X15000 (d), X8000 (e), X12000 (f), X 15000 (g), and X8000 (h). Uranyl acetate-lead citrate.
The stereocilia of the outer and inner hair cells were examined in Group III. Junctional complexes were observed at the apical part of outer hair cells, and outer phalangeal cells were surrounding the basal part of the outer hair cells. The cytoplasm of outer hair cells was homogenous, and mitochondria were lined up in a row along the lateral side of the cell. Lysosomes were located at the apical part of inner hair cells, and intercellular junction was intact in this group (Figures 4e, 4f).
The outer hair cells showed a normal ultrastructural morphology with an intact cell membrane and nuclei in Group V. The cuticular plate was observed in the apical part of the outer hair cells and nerve fibers, and the synaptic button was examined at the basal part of the cells in this group (Figures 4g, 4h).
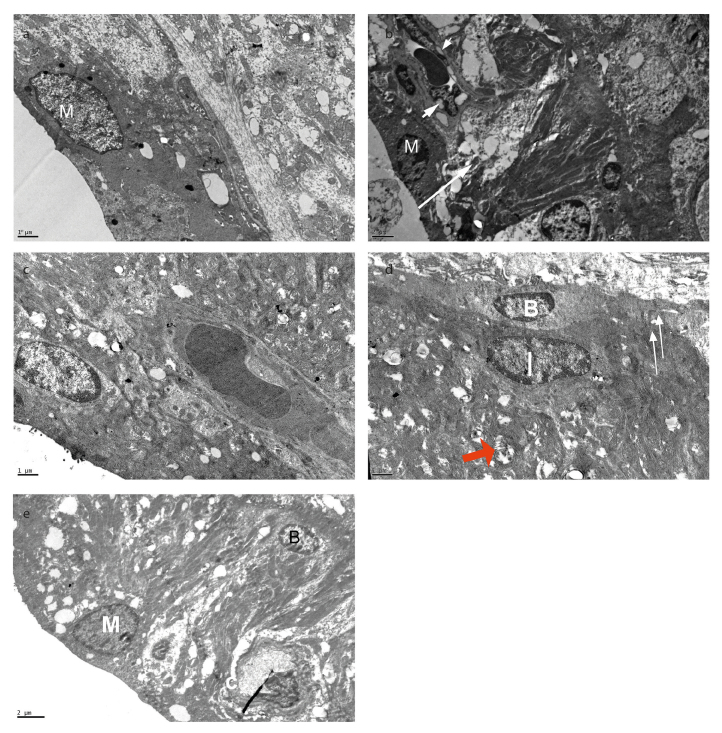
Marginal cells with a convex free surface and short microvilli were observed at the luminal surface of stria vascularis. Marginal cells with dark cytoplasm overlying the intermediate cells were observed in Group I. Basal and lateral infoldings of the marginal cells were sparse, and vacuoles were examined in the cytoplasm in Group I (CDDP+IP FA) (Figure 5a). Cisplatin treatment resulted in degenerative changes in the cytoplasm of intermediate cells. Basal cells could not be distinguished in this area in Group II (Figure 5b). Vacuoles were examined in the cytoplasm of endothelial cells in the intraepithelial capillary. The basal lamina under the endothelium was thick in this group
Figure 5. a–e.
Marginal cells with vacuoles and sparse basal infoldings in Group I (CDDP+IP FA). Empty spaces in the cytoplasm of intermediate cells (arrow), vacuoles in the cytoplasm of endothelial cell (arrowhead) in Group II (CDDP). Short microvilli and numerous mitochondria, basal infoldings of marginal cells and cristalysis in the mitochondria (red arrow) of Group III (CDDP+IT FA). Vacuoles in the lateral and basal foldings of marginal cells in Group V. M, marginal cell; I, intermediate cell; B, basal cell; C, capillary. Group I (CCDP+IP FA) (a), Group II (CDDP) (b), Group III (CCDP+IT FA) (c, d), Group V (IT FA) (e). X12000 (a), X6000 (b), X12000 (c), X12000 (d), and X8000 (e). Uranyl acetate-lead citrate.
Short microvilli and numerous mitochondria, and basal infoldings of marginal cells, were examined in Group III. Degeneration in the crista of mitochondria and vacuoles was detected in some areas in this group. Flattened basal cells located at the boundary of the epithelium were examined. Intermediate cells with branching cytoplasmic processes and homogenous cytoplasm were observed over the basal cells. Junctional complexes were observed between the cytoplasmic processes of the basal cell and intermediate cells in this group (Figures 5c, 5d).
Vacuoles were observed in the lateral and basal foldings of marginal cells in Group V (Figure 5e). The cytoplasm of endothelial cells in the intra-epithelial capillary showed a normal morphology in this group.
DISCUSSION
The mechanisms underlying cisplatin-induced ototoxicity and the resulting hearing loss have been studied by many researchers for a long time.
The present study investigated the effects of folic acid as a neuroprotective and antioxidant agent [9–11] on cisplatin-induced ototoxicity based on the previous reports [15, 16], showing direct effects of folic acid on vascular functions and oxidative stress via endothelial nitric oxide synthase.
To date, many studies have been conducted to prevent or reduce ototoxic effects of cisplatin. The agents used for otoprotective purposes in these studies were administered systemically or locally, and these studies were aimed at reducing or eliminating free radicals or increasing antioxidant substances.
Cadoni et al. [13] found low serum folate levels in a study conducted on 43 patients with sudden sensorineural hearing loss. All these patients had elevated homocysteine levels, low serum folate levels that were not associated with age, gender, smoking, alcohol use, and hypertension. The hearing loss was associated with a decreased antioxidant capacity and homocysteine metabolism as a result of low serum folate levels. In another experimental study, Stanger and Wonisch suggested that favorable effects of folic acid were not only related to the decrease in homocysteine levels, but also to direct free radical scavenging and antioxidant properties of folic acid that are independent from homocysteine levels, and they revealed that folic acid exerted direct effects on the vascular function and oxidative stress via endothelial nitric oxide synthase [8]. Yu et al. [11] demonstrated neuroprotective effects of folic acid in a rat cortical neuron culture, and they suggested that this effect is caused by anti-apoptotic feature of folic acid. Yilmaz et al. [17] used folic acid 10 mg/kg to experimentally induce diabetic peripheral neuropathy in rats and evaluate the effects of folic acid on diabetic peripheral neuropathy. They showed that folic acid decreased lipid peroxidation and exerted favorable effects on peripheral neuropathy through its antioxidant and neuroprotective properties.
At the doses administered in the present study, cisplatin caused increases in all ABR thresholds (4 kHz, 8 kHz, 12 kHz, and 16 kHz), IP folic acid provided significant protection against cisplatin-induced ototoxicity at 4 kHz, 8 kHz, and 16 kHz, and IP folic acid suppressed ototoxic effects of cisplatin at 12 kHz, although this was not found to be statistically significant. IT folic acid provided statistically significant protection against cisplatin-induced ototoxicity at 12 kHz and suppressed cisplatin-induced ototoxicity at 4 kHz, 8 kHz, and 16 kHz, although the effects at these frequencies were not statistically significant. In cisplatin-induced ototoxicity, damage to external hair cells, damage to marginal cells in stria vascularis and spiral ligament injury, and damage to the cells of spiral ganglia have been observed at the cellular level, [5] parallel to our findings. The cells of the organ of Corti, stria vascularis, and spiral ganglion showed a partially preserved morphology in both Group I and Group III. Our histopathological findings supported the ABR measurement results. The present study found that IP folic acid caused a significant increase in plasma folic acid levels, which suggests that IP folic acid passes into the circulation at sufficient amounts, and IT folic acid does not cause a significant increase in plasma folic acid levels. The present study found a decrease in plasma homocysteine levels of rats that received IT folic acid, although this was not statistically significant; this finding suggests that folic acid may not have effects only on the homocysteine metabolism, but that it may have other effects independent from homocysteine levels. We found a statistically significant increase in plasma homocysteine levels of rats that received cisplatin and physiological saline (Group II). Further studies are required to determine whether or not this increase was associated with cisplatin administration
CONCLUSION
Our study results suggest that folic acid has otoprotective effects on cisplatin-induced ototoxicity and that it is a promising otoprotective agent for routine use in cisplatin-induced ototoxicity. However, further studies are required to elucidate its mechanisms of action, appropriate dosing, perfect timing of administration, and whether it has anti-tumoricidal effects. This is the first study to evaluate the protective effects of folic acid on cisplatin-induced ototoxicity.
Footnotes
This study was presented at the “15th Internatinal Congress of Histochemistry and cytochemistry “From molecules to diseases” ICHC 2017 and 4th National Congress of Otology and Neurotology”, “18–21 May 2017 and 21–24 April 2016, “Antalya, Turkey”.
Ethics Committee Approval: This study was approved by the ethical committee of Ankara Training and Research Hospital (Date: 04.06.2015, number: 370).
Informed Consent: N/A
Peer-review: Externally peer-reviewed.
Author Contributions: Concept –T.T.T., H.K., I.A., N.D.Z..; Design - T.T.T., H.K. I.A., O.G., C.S., M.E.C., Z.K., H.U., N.A., N.D.Z.; Supervision – T.T.T., H.K., I.A., O.G., C.S., M.E.C., Z.K., H.U., N.A., N.D.Z..; Resource - T.T.T., H.K., I.A., O.G., C.S., M.E.C., Z.K., H.U., N.A., N.D.Z .; Materials - T.T.T., H.K., I.A., O.G., C.S. N.D.Z. .; Data Collection and/or Processing - T.T.T., I.A., O.G., C.S., N.D.Z .; Analysis and/or Interpretation - T.T.T., I.A., O.G., C.S., N.D.Z.; Literature Search - T.T.T., H.K., I.A., M.E.C., Z.K., H.U., N.A., N.D.Z.; Writing - T.T.T., I.A., N.D.Z.; Critical Reviews - H.K., I.A., N.A., N.D.Z.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: This research was supported by a Grant of the Research Foundation of Ministry of Health, Ankara Training and Research Hospital, Turkey.
REFERENCES
- 1.Matz GJ. The ototoxic effects of ethacrynic acid in man and animals. Laryngoscope. 1976;86:1065–86. doi: 10.1288/00005537-197608000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Kavanagh KT, McCabe BF. Ototoxicity of oral neomycin and vancomycin. Laryngoscope. 1983;93:649–53. doi: 10.1002/lary.1983.93.5.649. [DOI] [PubMed] [Google Scholar]
- 3.Schweitzer VG. Ototoxicity of chemotherapeutic agents. Otolaryngol Clin North Am. 1993;26:759–89. [PubMed] [Google Scholar]
- 4.Friedman RA, House JW. Profound hearing loss associated with hydrocodone/acetaminophen abuse. Am J Otol. 2000;21:188–91. doi: 10.1016/S0196-0709(00)80007-1. [DOI] [PubMed] [Google Scholar]
- 5.Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechansims of cisplatin-induced ototoxicity and prevention. Hear Res. 2007;226:157–67. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Rybak LP, Mukherjea D, Jajoo S, Ramkumar V. Cisplatin ototoxicity and protection: clinical and experimental studies. Tohoku J Exp Med. 2009;219:177–86. doi: 10.1620/tjem.219.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van den Berg JH, Beijnen JH, Balm AJ, Schellens JH. Future opportunities in preventing cisplatin induced ototoxicity. Cancer Treat Rev. 2006;32:390–7. doi: 10.1016/j.ctrv.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Stanger O, Wonisch W. Enzymatic and non-enzymatic antioxidative effects of folic acid and its reduced derivates. Subcell Biochem. 2012;56:131–61. doi: 10.1007/978-94-007-2199-9_8. [DOI] [PubMed] [Google Scholar]
- 9.Das UN. Folic acid says NO to vascular diseases. Nutrition. 2003;19:686–92. doi: 10.1016/S0899-9007(02)01044-4. [DOI] [PubMed] [Google Scholar]
- 10.Das UN. Folic acid and polyunsaturated fatty acids improve cognitive function and prevent depression, dementia, and Alzheimer’s disease - but how and why? Prostaglandins Leukot Essent Fatty Acids. 2008;78:11–9. doi: 10.1016/j.plefa.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Yu HL, Li L, Zhang XH, Xiang L, Zhang J, Feng JF, et al. Neuroprotective effects of genistein and folic acid on apoptosis of rat cultured cortical neurons induced by beta-amyloid 31–35. Br J Nutr. 2009;102:655–62. doi: 10.1017/S0007114509243042. [DOI] [PubMed] [Google Scholar]
- 12.Berner B, Odum L, Parving A. Age-related hearing impairment and B-vitamin status. Acta Otolaryngol. 2000;120:633–7. doi: 10.1080/000164800750000469. [DOI] [PubMed] [Google Scholar]
- 13.Cadoni G, Agostino S, Scipione S, Galli J. Low serum folate levels: a risk factor for sudden sensorineural hearing loss? Acta Otolaryngol. 2004;124:608–11. doi: 10.1080/00016480410016216. [DOI] [PubMed] [Google Scholar]
- 14.Durga J, Verhoef P, Anteunis LJ, Schouten E, Kok FJ. Effects of folic acid supplementation on hearing in older adults: a randomized, controlled trial. Ann Intern Med. 2007;146:1–8. doi: 10.7326/0003-4819-146-1-200701020-00003. [DOI] [PubMed] [Google Scholar]
- 15.Doshi SN, McDowell IF, Moat SJ, Payne N, Durrant HJ, Lewis MJ, et al. Folic acid improves endothelial function in coronary artery disease via mechanisms largely independent of homocysteine lowering. Circulation. 2002;105:22–6. doi: 10.1161/hc0102.101388. [DOI] [PubMed] [Google Scholar]
- 16.Antoniades C, Shirodaria C, Warrick N, Cai S, de Bono J, Lee J, et al. 5-Methyl-tetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and eNOS coupling. Circulation. 2006;114:1193–201. doi: 10.1161/CIRCULATIONAHA.106.612325. [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz M, Aktug H, Oltulu F, Erbas O. Neuroprotective effects of folic acid on experimental diabetic peripheral neuropathy. Toxicol Ind Health. 2016;32:832–40. doi: 10.1177/0748233713511513. [DOI] [PubMed] [Google Scholar]