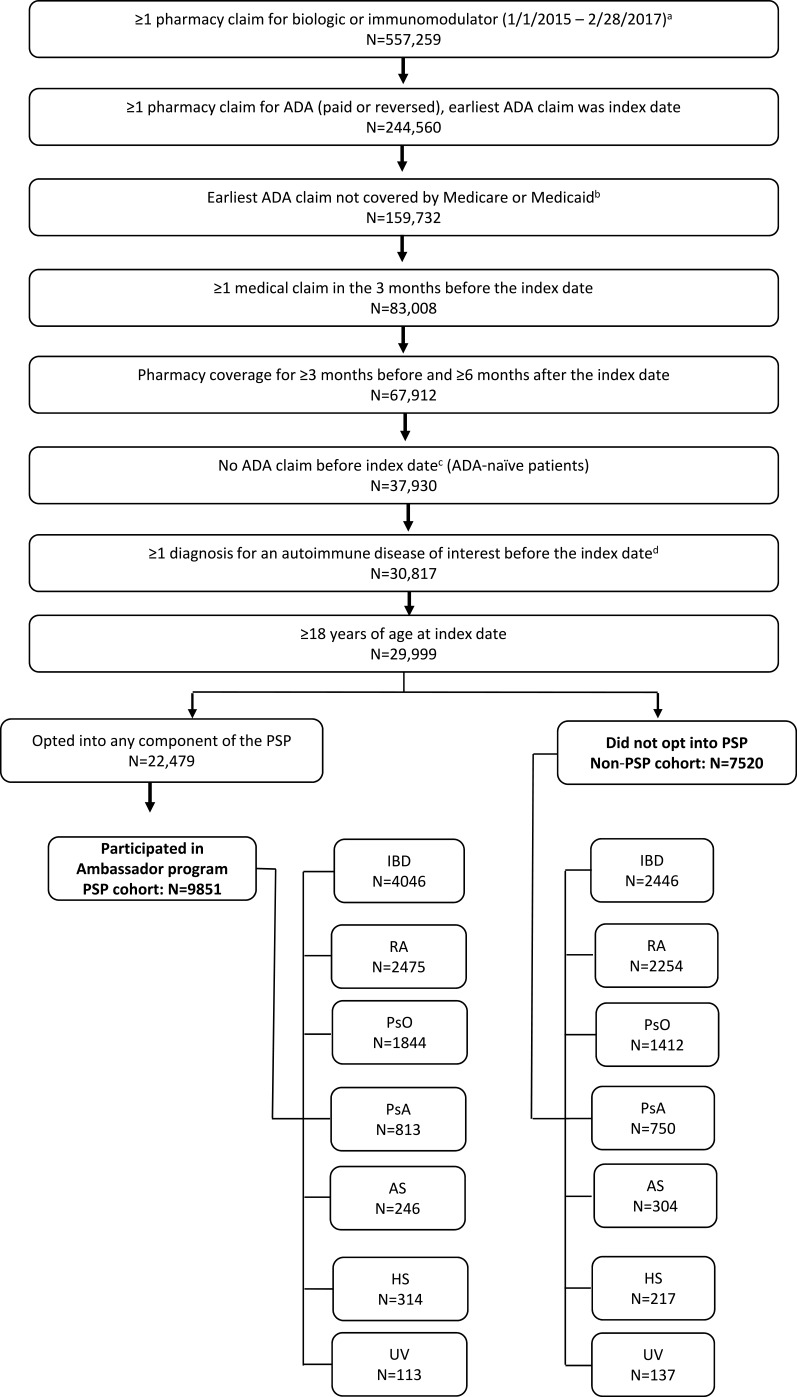
Figure 1.
Study sample selection.
Notes: aDirect feed claims consisted of filled claims only and were excluded from abandonment analysis. The following treatments were included: etanercept, infliximab, certolizumab, golimumab, anakinra, tocilizumab, secukinumab, ixekizumab, ustekinumab, abatacept, rituximab, natalizumab, vedolizumab, tofacitinib, and apremilast. bClaims with government-provided insurance were excluded as these patients were prohibited from using all components of the PSP. cDetermined using all available claims from 1/1/2006 to the index date. dAutoimmune diseases included rheumatoid arthritis, Crohn’s disease, ulcerative colitis, psoriasis, psoriatic arthritis, ankylosing spondylitis, hidradenitis suppurativa, and uveitis.
Abbreviations: ADA, adalimumab; AS, ankylosing spondylitis; HS, hidradenitis suppurativa; IBD, inflammatory bowel disease; PsA, psoriatic arthritis; PsO, psoriasis; PSP, patient support program; RA, rheumatoid arthritis; UV, uveitis.