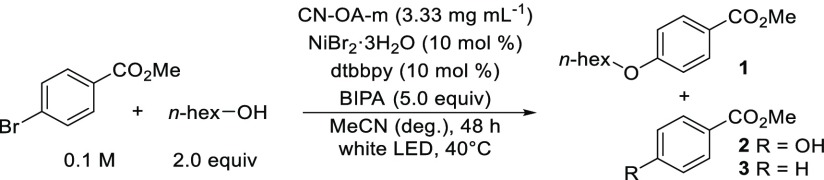
Table 1. Optimized Conditions and Control Experimentsa.
| entry | conditions | 1 (%)b | 2 (%)b | 3 (%)b |
|---|---|---|---|---|
| 1 | as shown | 96 | 3 | 1 |
| 2 | CN-OA-m (1.66 mg mL–1) | 90 | 6 | n.d. |
| 3 | 24 h | 55 | 3 | n.d. |
| 4 | 24 h with 10 mol % quinuclidine | 61 | 2 | n.d. |
| 5 | methyl 4-chlorobenzoate | 4 | n.d. | n.d. |
| 6 | no light | n.d. | n.d. | n.d. |
| 7 | no CN-OA-m | n.d. | n.d. | n.d. |
| 8 | no NiBr2·3H2O | n.d. | n.d. | n.d. |
| 9 | no dtbbpy | n.d. | n.d. | n.d. |
| 10 | no BIPA | n.d. | n.d. | n.d. |
| 11 | no degassing | n.d. | n.d. | n.d. |
Reaction conditions: methyl 4-bromobenzoate (0.3 mmol), 1-hexanol (0.6 mmol), CN-OA-m (10 mg), NiBr2·3H2O (30 μmol), dtbbpy (30 μmol), BIPA (1.5 mmol), MeCN (3.0 mL), white LEDs at 40 °C for 48 h.
Determined by 1H NMR using 1,3,5-trimethoxybenzene as internal standard. n.d. = not detected.