Figure 4.
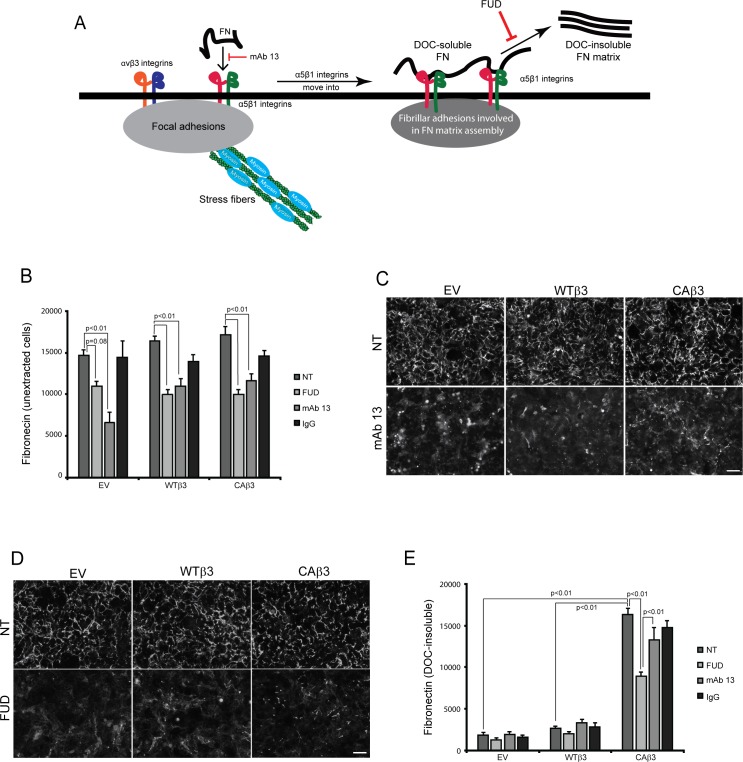
Both mAb 13 and FUD inhibit fibronectin fibril formation. (A) Schematic showing the canonical α5β1 integrin–mediated pathway involved in fibronectin fibrillogenesis.58 As previously reported,46 both α5β1 and αvβ3 integrins can colocalize to focal adhesions in TM cells. During the initial stages of fibril formation, soluble fibronectin dimers bound to α5β1 integrins are unfolded within focal adhesions together with αvβ3 integrins. The fibronectin–α5β1 integrin complex, but not αvβ3 integrin, is then translocated to regions of fibril formation called fibrillar adhesions.58 While in fibrillar adhesions, fibronectin–fibronectin binding interactions promote the assembly of DOC-insoluble fibrils. Both the β1 integrin function-blocking mAb 13 and FUD inhibit this process as indicated in the schematic. (B) OCW analysis of unextracted confluent monolayers of EV, WTβ3, and CAβ3 cells. Cells were left untreated (NT) or treated for 24 hours with 500 nM FUD, 10 μg/mL mAb 13, or control IgG. Monoclonal Ab 13 significantly reduced fibronectin fibril formation in all three cell lines (P < 0.01) while FUD significantly reduced fibril formation in the WTβ3 and CAβ3 cells, respectively (P < 0.01). The ∼25% reduction in response to FUD seen in the EV cultures was not statistically significant (P = 0.08). Data are pooled from two separate assays with triplicate determinations (n = 6) and represent the mean ± SE. (C) Immunofluorescence images of intact, unextracted monolayers of EV, WTβ3, and CAβ3 cells that were left untreated or treated with 10 μg/mL mAb 13 for 24 hours prior to labeling with rabbit fibronectin antiserum. Monoclonal Ab 13 disrupted fibronectin fibril formation in all three cell lines. The labeling is a combination of both soluble and insoluble fibronectin fibrils. Scale bar: 50 μm. (D) Immunofluorescence images of intact, unextracted confluent monolayers of EV, WTβ3, and CAβ3 cells that were left untreated or treated with 500 nM FUD for 24 hours prior to labeling with rabbit fibronectin antiserum. FUD disrupted fibronectin fibril formation in all three cell lines. The labeling is a combination of both soluble and insoluble fibronectin fibrils. Scale bar: 50 μm. (E) OCW analysis of DOC-extracted monolayers of EV, WTβ3, and CAβ3 cells. Cells were treated as in (B) prior to DOC extraction; mAb 13 did not have an effect on DOC-insoluble fibronectin levels in any of the three cell lines. FUD only statistically significantly decreased DOC-insoluble fibronectin levels in the CAβ3 cell cultures relative to both untreated cells and cells treated with mAb 13 (both P < 0.01). The minor decreases in the insoluble fibronectin matrices seen in both EV and WTβ3 cells were not statistically significant. Comparing untreated groups, CAβ3 cells exhibited significantly more DOC-insoluble fibronectin matrix than either EV or WTβ3 cells, respectively (P < 0.01). Data are pooled from two separate assays with triplicate determinations (n = 6) and represent the mean ± SE.