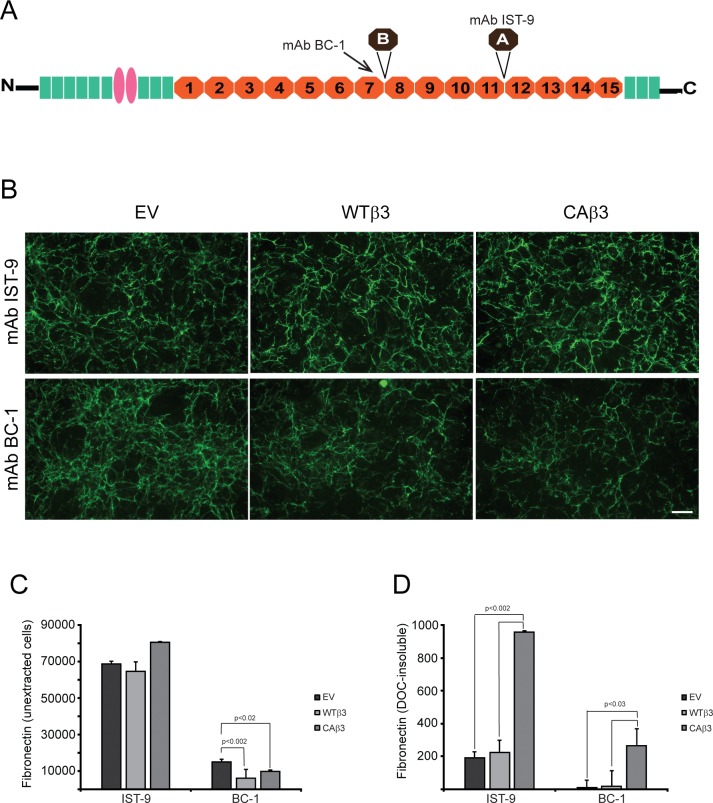
Figure 10.
Constitutively active αvβ3 integrin increases deposition of specific fibronectin isoforms into the DOC-insoluble matrix. (A) Fibronectin schematic showing the locations of the EDA and EDB alternatively spliced domains. The mAb BC-1 recognizes a conformation-specific epitope formed in the seventh type III repeat when the EDB domain is present.85 The IST-9 mAb detects a sequence found within the EDA domain. (B) Intact, confluent monolayers of EV, WTβ3, and CAβ3 cells were immunolabeled with mAbs IST-9 and BC-1, respectively. No clear differences in labeling intensity were observed between the three cell lines for mAb IST-9. EV cells, however, demonstrated more intense labeling with mAb BC-1 than both WTβ3 and CAβ3 cells. Negative control cells were labeled with mAb GAL-13 against β-galactosidase and showed no significant labeling (not shown). This experiment was performed twice with identical results. Scale bar: 50 μm. (C) OCW analysis of intact, unextracted monolayers of EV, WTβ3, and CAβ3 cells plated for 24 hours. No significant difference was found between any of the three cell lines with respect to EDA+ fibronectin. Both WTβ3 cells (P < 0.002) and CAβ3 cells (P < 0.02) demonstrated less EDB+ fibronectin in intact monolayers relative to EV cells. Results represent the mean ± SE and consist of data pooled from two assays using quadruplicate and triplicate determinations, respectively (n = 7). (D) OCW analysis of DOC-insoluble fibronectin fibrils in EV, WTβ3, and CAβ3 cultures. CAβ3 cells demonstrated significantly higher levels of DOC-insoluble EDA+ fibronectin fibrils than EV and WTβ3 cells (P < 0.002). CAβ3 cells demonstrated significantly higher levels of DOC-insoluble EDB+ fibronectin fibrils than EV and WTβ3 cells (P < 0.03). Results represent the mean ± SE and consist of data pooled from two assays using triplicate determinations (n = 6).