Abstract
Breast cancer is the most frequently diagnosed cancer in women globally. Genetic mutations can increase the risk of developing breast cancer. Inherited germline mutations in BRCA1 and BRCA2 tumor suppressor genes (gBRCAm) account for 5% to 10% of breast cancer cases. The recent approval of olaparib, a poly (ADP-ribose) polymerase (PARP) inhibitor, in HER2-negative, metastatic breast cancer provides an additional treatment option for patients with a gBRCAm. Inhibition of PARP results in the trapping of the PARP-DNA complex at replication forks, causing single-strand breaks to become double-strand breaks (DSBs). PARP trapping and the accumulation of DSBs ultimately leads to cell apoptosis. Cells deficient in BRCA1/2 are particularly sensitive to the effects of PARP inhibition, as cells lacking these functional proteins are unable to repair DSBs, resulting in synthetic lethality. The phase III OlympiAD trial showed a progression-free survival benefit but no overall survival benefit, leading to the US Food and Drug Administration approval of olaparib. The purpose of this article is to describe current data regarding the use of olaparib in metastatic breast cancer, its role in the treatment of patients with a gBRCAm, and the clinical implications of its approval for oncology advanced practitioners.
Breast cancer is the most frequently diagnosed cancer in women globally. In 2019, an estimated 268,600 new cases will be diagnosed in the United States. The rate of newly diagnosed cases has largely remained the same over the past several years, with death rates falling an average of 1.8% each year since 2006 (American Cancer Society, 2019). The treatment of breast cancer is generally dependent on the expression of estrogen and progesterone hormone receptors (HR) and the amplification of human epidermal growth factor receptor 2 (HER2) proteins on tumor cells. Targeted therapies (i.e., estrogen receptor antagonists, aromatase inhibitors, and anti-HER2 therapies) have drastically improved survival rates in patients with HR-positive and/or HER2-positive disease (Ballinger, Meier, & Jansen, 2018). Approximately 15% to 20% of breast cancers lack expression of estrogen and progesterone receptors and HER2 gene amplification, also known as triple-negative breast cancer (TNBC). The aggressive nature of TNBC can be attributed to early age at presentation, advanced-stage disease, higher prevalence of genetic mutations, and limited treatment options, with most patients relapsing within 1 to 2 years of initial presentation. Additionally, patients with TNBC have the worst disease-free and overall survival (OS) rates of all breast cancer types, with only 30% of patients living 5 years after diagnosis (Guney Eskiler, Cecener, Egeli, & Tunca, 2018). Until recently, there has been little advancement in the treatment of TNBC.
Certain genetic mutations can greatly increase the risk of developing breast cancer. Specifically, germline mutations in BRCA1 and BRCA2 tumor suppressor genes (gBRCAm) account for 5% to 10% of breast cancer cases (Godet & Gilkes, 2017). The lifetime risk of developing breast cancer in BRCA1 and BRCA2 mutation carriers is 72% and 68%, respectively, compared to 12% in noncarriers (National Cancer Institute, 2018). BRCA1 and BRCA2 are tumor suppressor genes responsible for the repair of double-strand DNA breaks (DSBs), an important step in the DNA repair pathway. Cells lacking functional BRCA genes rely on less accurate repair mechanisms, resulting in more genomic instability and an increased risk of developing certain types of cancers, including breast, ovarian, fallopian tube, primary peritoneal, prostate, and pancreatic cancers. An estimated 75% of patients with TNBC are carriers of a BRCA1 or BRCA2 gene mutation (Balmaña, Díez, & Castiglione, 2009). Although screening for the BRCA1 or BRCA2 gene mutation is not currently recommended in the general population, certain patients with an individual or family history may benefit from early screening. Patients with an increased risk of harboring a BRCA mutation include having a breast cancer diagnosis before the age of 50, bilateral breast cancer, both breast and ovarian cancers in either the same woman or the same family, multiple breast cancers in the family, two or more primary types of BRCA1- or BRCA2-related cancer in a single family member, male breast cancer, or Ashkenazi Jewish ethnicity (U.S. Preventive Services Task Force, 2013).
Metastatic breast cancer accounts for 6% of all initial diagnoses, with a 5-year OS rate of 27% (National Comprehensive Cancer Network [NCCN], 2018; Surveillance, Epidemiology, and End Results Program, 2018). Current treatment options for patients with metastatic TNBC are limited to single-agent chemotherapy with one of the following preferred agents: doxorubicin, liposomal doxorubicin, paclitaxel, eribulin, capecitabine, gemcitabine, and vinorelbine (NCCN, 2018). On January 8, 2018, the US Food and Drug Administration (FDA) granted regular approval of olaparib (Lynparza) for patients with gBRCAm, HER2-negative metastatic breast cancer (FDA, 2018). The purpose of this article is to describe current data regarding the use of olaparib in metastatic breast cancer, its role in the treatment of patients with a gBRCAm, and the clinical implications of its approval for oncology advanced practitioners.
PHARMACOLOGY AND MECHANISM OF ACTION
Cells are regularly exposed to radiation, ultraviolet light, or chemicals that routinely cause DNA damage. Cell survival is dependent on DNA repair pathways to maintain homeostasis and genomic stability. BRCA1 and BRCA2 tumor suppressor genes play an important role in the DNA repair pathway and are responsible for the repair of DSBs. Mutations in either of these genes result in the accumulation of DSBs, causing the genomic instability thought to be responsible for the development of some cancers (Dziadkowiec, Gąsiorowska, Nowak-Markwitz, & Jankowska, 2016).
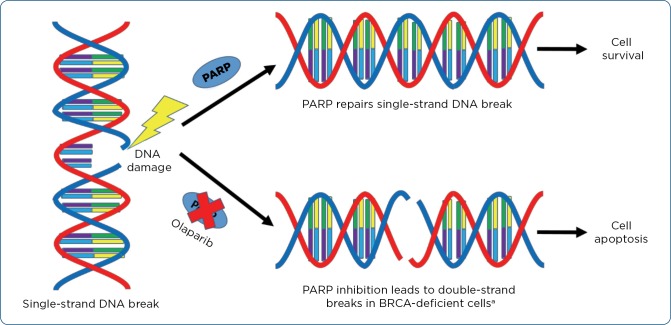
Poly (ADP-ribose) polymerases (PARPs) are a group of enzymes activated by DNA damage. PARP1 and PARP2 assist in the repair of single-strand breaks (SSBs) through base excision repair. Inhibition of PARP results in the trapping of the PARP-DNA complex at replication forks, causing SSBs to become DSBs. PARP trapping and the accumulation of DSBs ultimately lead to cell apoptosis if not corrected by appropriate repair mechanisms (see Figure 1). Cells deficient in BRCA1/2 are particularly sensitive to the effects of PARP inhibition, as cells lacking these functional proteins are unable to repair DSBs, resulting in synthetic lethality. Synthetic lethality occurs when a cell can survive either PARP inhibition or BRCA mutation; however, the combination results in cell death targeting the tumor cells with BRCA mutations over normal cells. Since initial approval in December 2014, olaparib and other PARP inhibitors have quickly established their role in the treatment of advanced ovarian cancer, another malignancy commonly associated with BRCA1/2 mutations. The recent approval of olaparib in HER2-negative, metastatic breast cancer offers an additional treatment option for patients with a gBRCAm (Dziadkowiec et al., 2016).
Figure 1.
Olaparib mechanism, specifically in BRCA-deficient cells compared to normal cells. PARP = poly (ADP-ribose) polymerase; BRCA = breast cancer gene 1. aBRCA-proficient cells can repair double-strand breaks, resulting in cell survival.
CLINICAL TRIALS
Olaparib showed promise in a phase I trial evaluating its use in patients with solid tumors refractory to standard treatment. Initially, patients were not required to be BRCA mutation carriers. The objectives of the study were to evaluate safety, adverse-event profile, dose-limiting toxicity, maximum-tolerated dose, dose at which PARP is maximally inhibited, and pharmacokinetic and pharmacodynamics profiles. An accelerated titration design was used during the dose-escalation phase, which determined the maximum-tolerated dose of olaparib to be 400 mg twice daily. Grade 3 mood alteration, grade 4 thrombocytopenia in a patient recently treated with chemotherapy, and grade 3 somnolence were all noted as the dose-limiting toxicities. Rates of grade ≥ 3 adverse events (AEs) were low (≤ 5%) and included anemia, lymphopenia, nausea, vomiting, fatigue, and dizziness. Additional AEs noted were dysgeusia, anorexia, dyspepsia, diarrhea, and stomatitis. The inhibition of PARP at 90% was seen in patients treated with 60 mg or more of olaparib twice daily. During the expansion phase, only BRCA1 or BRCA2 mutation carriers were enrolled to evaluate the antitumor activity of olaparib 200 mg twice daily. Of the 19 BRCA mutation carriers with breast, ovarian, or prostate cancer evaluated for response to olaparib, 12 (63%) had a clinical benefit with radiologic or tumor marker response, or disease stabilization. This study established the benefit of olaparib and paved the way for additional clinical trials (Fong et al., 2009).
The ICEBERG study was a phase II, nonrandomized sequential cohort, proof-of-concept trial designed to evaluate the efficacy, safety, and tolerability of olaparib in patients with a BRCA1/2 mutation and advanced breast cancer. Patients were required to have locally advanced or metastatic breast cancer with one or more measurable lesions and a germline BRCA1/2 mutation. A total of 54 patients, including 7 (13%) with HER2-positive disease, were assigned in a nonrandomized fashion to one of two dosing cohorts. Cohort 1 (olaparib at 400 mg twice daily) was selected based on the maximum-tolerated dose established in the phase I study. Cohort 2 (olaparib at 100 mg twice daily) was selected as a lower dose, which also showed activity in the phase I study (Fong et al., 2009). The primary endpoint evaluated was objective response rate (ORR), and secondary endpoints included clinical benefit rate (CBR, defined as the percentage of patients with complete response, partial response, and stable disease for ≥ 23 weeks), progression-free survival (PFS), and duration of response. A total of 54 patients were enrolled in the trial, with 29 patients completing the full study schedule, receiving olaparib for ≥ 168 days (Tutt et al., 2010).
The ORR in the intention-to-treat population was higher in cohort 1 than in cohort 2 (41% vs. 22%, respectively). The CBR was higher for cohort 1 than cohort 2 (52% vs. 26%, respectively). Median PFS was longer in cohort 1 than cohort 2 (5.7 months vs. 3.8 months, respectively). Median duration of response was similar between the two cohorts (144 days vs. 141 days, respectively; Tutt et al., 2010).
Adverse events were reported in 44 patients (81%), with the majority of these events being grade 1/2. The most common AEs reported were nausea, fatigue, anemia, vomiting, anorexia, and diarrhea. Only 13 patients (24%) had AEs that were grade 3 or 4, which were similar in both groups. Grades 3 or 4 AEs were more common in the 400 mg group and included nausea (15%), fatigue (15%), vomiting (11%), and anemia (11%) whereas the 100 mg group had only anemia (7%), fatigue (4%), and anorexia (4%). The results of this study supported the benefit of using olaparib in BRCA-mutated, HER2-negative breast cancers, prompting further studies to evaluate effectiveness as compared to the standard of care with cytotoxic chemotherapy (Tutt et al., 2010).
OlympiAD was an international, open-label, multicenter, randomized phase III trial that evaluated the efficacy and safety of olaparib in patients with metastatic HER2-negative and either estrogen receptor (ER)/progesterone receptor (PR)–positive or –negative breast cancer. Patients were required to have a known or suspected gBRCAm and received no more than two previous chemotherapy regimens. A total of 302 patients were randomized in a 2:1 fashion to olaparib at 300 mg twice daily or single-agent chemotherapy of provider’s choice, including eribulin, capecitabine, or vinorelbine (Robson et al., 2017). Of note, a pharmacokinetic study determined that olaparib tablets (available as 100 mg and 150 mg) have improved bioavailability compared to the capsule formulation (available as 50 mg). Based on the results of this study, steady-state exposure was 77% higher with olaparib tablets at a dose of 300 mg twice daily than the capsules at a dose of 400 mg twice daily. Due to lower pill burden and better bioavailability, the tablet formulation and dosing were selected for the OlympiAD trial (AstraZeneca Pharmaceuticals LP, 2018).
The primary endpoint of this study evaluated PFS using Modified Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 or death from any cause. Secondary endpoints included OS, safety outcomes, and ORR. This study demonstrated PFS was significantly longer in the olaparib group compared to the standard-therapy group (7.0 months vs. 4.2 months, p < .001). Overall survival did not differ significantly between the two groups, with 19.3 months in the olaparib group vs. 19.6 months in the standard therapy group (p = 0.57). Objective response rate was doubled in the olaparib group compared to the standard therapy group (59.9% vs. 28.8%). Although this study was not powered to detect a difference in subgroups, there was a benefit seen in patients with TNBC in the olaparib group (Robson et al., 2017).
In the OlympiAD trial, the majority of patients (97%) treated with olaparib experienced an adverse event (AE) of any grade. Many experienced AEs that were grade 1/2 (61%). The olaparib group had a lower rate of grade 3/4 AEs compared to the standard therapy group (37% vs. 51%). The most common AEs (occurring in > 20% of patients) in the olaparib group were anemia, neutropenia, nausea, vomiting, diarrhea, and fatigue (Table 1). The only grade 3/4 AE reported at > 10% was anemia (16%). Adverse events reported in the OlympiAD trial were similar to those reported in previous studies. Dose reductions due to AEs occurred in 25% of patients. Dose reductions in the olaparib group occurred most often due to anemia, which occurred in 14% of patients. Treatment delays or interruptions occurred in 35% of patients. Discontinuation of olaparib occurred in 5% of patients due to anemia, thrombocytopenia, increased intracranial pressure, abdominal pain, dyspnea, and erythema nodosum (Robson et al., 2017). Overall, olaparib appears to be generally well tolerated. These results led to the FDA approval of olaparib in metastatic HER2-negative breast cancer in patients with a known or suspected gBRCAm who have received previous treatment with chemotherapy (AstraZeneca Pharmaceuticals LP, 2018).
Table 1.
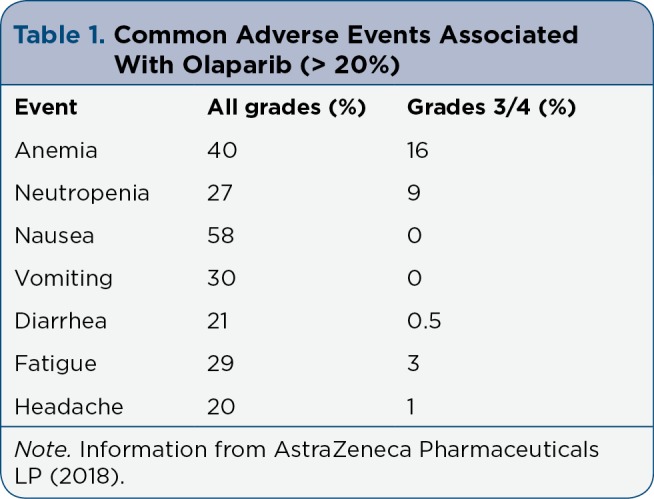
Common Adverse Events Associated With Olaparib (> 20%)
Additional studies are looking at the benefit of olaparib in combination with cytotoxic chemotherapy. A phase I, open-label, multicenter study evaluated the safety and tolerability of olaparib in combination with paclitaxel for first- or second-line treatment in metastatic TNBC (Dent et al., 2013). All patients received olaparib at 200 mg twice daily in combination with paclitaxel 90 mg/m² as an IV infusion on days 1, 8, and 15 of a 28-day cycle. Due to a greater-than-expected rate of grade ≥ 2 neutropenia within the first two cycles of treatment, a protocol amendment allowed for a second cohort of patients to be enrolled. Patients in cohort 2 received the same dosing as patients in cohort 1, with the addition of prophylactic granulocyte colony-stimulating factor (G-CSF) to help maintain optimal dose intensity of paclitaxel. The primary endpoints evaluated were safety and tolerability of the combination of olaparib and paclitaxel. Secondary endpoints evaluated were ORR and PFS.
When evaluating safety, the majority (84%) of patients experienced an AE related to treatment. Sixty-eight percent of patients experienced at least one ≥ grade 3 event, with more events occurring in cohort 1 than cohort 2 (89% vs. 50%, respectively). Grade ≥ 3 neutropenia was more common in cohort 1 than cohort 2 (44% vs. 20%, respectively). Dose reductions for paclitaxel were required in 89% of patients in cohort 1 vs. 60% in cohort 2.
Objective response rate was lower in cohort 1 than cohort 2 (33% vs. 40%, respectively), but PFS was similar between the two groups (6.3 months vs. 5.2 months). This study concluded there was a higher incidence of neutropenia when olaparib and paclitaxel were combined. There was evidence of efficacy; however, optimal dosing and schedule should be further evaluated to prevent hematologic toxicity (Dent et al., 2013).
ROLE IN THERAPY
Olaparib is the first treatment approved specifically for BRCA mutation carriers with HER2-negative metastatic breast cancer and previous treatment with chemotherapy in the neoadjuvant, adjuvant, or metastatic setting. Of note, patients with HR-positive disease should be treated with appropriate endocrine therapy or deemed inappropriate for endocrine therapy prior to the initiation of olaparib (FDA, 2018). The NCCN added olaparib to the breast cancer treatment guidelines in January 2018 and updated it to a Category 1 recommendation in the most recent guideline update in October 2018. Unfortunately, specific guidance regarding prior treatments as approved by the FDA is not included in the update (NCCN, 2018). Through the concept of synthetic lethality, olaparib causes death in BRCA-deficient cells while sparing healthy cells, a unique treatment concept in TNBC. The AE profile is tolerable, and oral dosing may be preferred in certain situations.
DOSING AND ADMINISTRATION
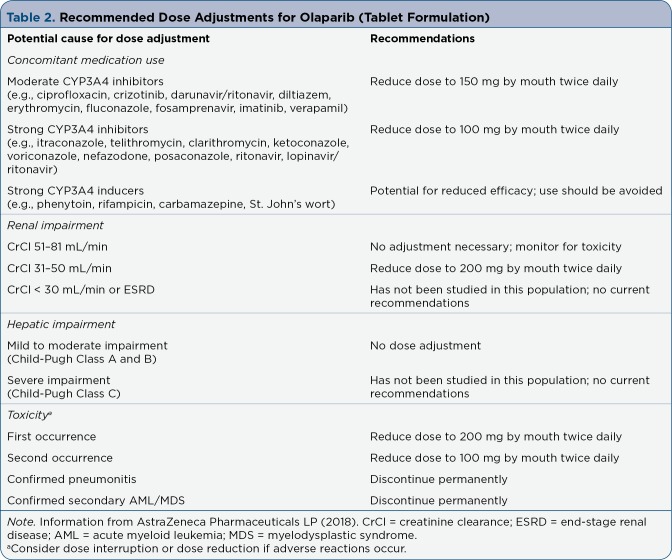
The recommended dose of olaparib tablets is 300 mg by mouth twice daily (12 hours apart) to be continued until disease progression or unacceptable toxicity. Olaparib can be administered with or without food and should be swallowed whole. Olaparib is available as 100-mg and 150-mg tablets. Of note, the 50-mg capsule formulation is no longer available from manufacturers. Only the tablet formulation is FDA-approved for the breast cancer indication and attention by providers should be noted (AstraZeneca Pharmaceuticals LP, 2018). The capsules cannot be substituted for tablets on a mg-per-mg basis, an important distinction between the formulations. Dose adjustments for renal and hepatic function can be found in Table 2 (AstraZeneca Pharmaceuticals LP, 2018). According to a pharmacokinetic evaluation, when olaparib is administered with a high-fat meal, there is a slowed rate of absorption, although it does not appear to significantly alter the extent of total absorption (AstraZeneca Pharmaceuticals LP, 2018).
Table 2.
Recommended Dose Adjustments for Olaparib (Tablet Formulation)
Olaparib is metabolized via hepatic CYP3A4 enzymes, primarily through oxidation with some metabolites undergoing further glucuronide or sulfate conjugation. Metabolites are excreted through both urine (44%) and feces (42%). The average time to peak concentration is 1.5 hours, and the mean terminal half-life is 14.9 hours (AstraZeneca Pharmaceuticals LP, 2018). All patients should be evaluated for drug interactions before starting olaparib. Concomitant use with moderate or strong CYP3A4 inducers should be avoided due to decreased efficacy of olaparib. Patients should avoid grapefruit juice and Seville oranges, which may increase olaparib plasma concentrations, resulting in increased toxicity. Detailed recommendations for olaparib dose adjustments are outlined in Table 2 (AstraZeneca Pharmaceuticals LP, 2018).
IMPLICATIONS FOR THE ADVANCED PRACTITIONER
Although BRCA mutations only occur in 5% to 10% of breast cancer diagnoses, treatment options are limited and often carry significant toxicity. The FDA approval of olaparib in patients with gBRCAm, HER2-negative metastatic breast cancer provides a targeted treatment alternative to cytotoxic chemotherapy (FDA, 2018).
Warnings for olaparib include the risk of myelodysplastic syndrome (MDS), acute myeloid leukemia (AML), and pneumonitis. Based on long-term follow-up, MDS/AML occurred in < 1.5% of patients. It is important to note that all patients who developed MDS or AML had previously received chemotherapy with platinum and alkylating agents, potentially a confounding factor. Pneumonitis occurred in < 1% of patients treated with olaparib, with some events resulting in death. Patients should also be informed of the potential risk of fetal harm, and women of reproductive potential should use effective contraception during and for at least 6 months following completion of treatment (AstraZeneca Pharmaceuticals LP, 2018).
Routine monitoring should include a complete blood count (CBC) at baseline and monthly thereafter or as clinically indicated. For patients with prolonged hematologic toxicity, CBC should be monitored weekly until recovery. Renal function, urine pregnancy test, and signs or symptoms of AML/MDS and pneumonitis should also be monitored periodically. Dose interruptions and dose reductions should be considered for patients with AEs (see Table 2; AstraZeneca Pharmaceuticals LP, 2018). Concomitant medications should be reviewed regularly to assess for potential drug-drug interactions. As with any oral therapy, compliance should be assessed on a regular basis to ensure optimal clinical outcomes.
ONGOING CLINICAL TRIALS
Currently, there are approximately 31 actively recruiting breast cancer trials involving olaparib. Several studies are investigating the use of olaparib in patients with somatic BRCA mutations as compared to gBRCAm, in combination with radiation therapy, and in the adjuvant setting after completion of neoadjuvant and local therapies. There are also several studies evaluating the use of olaparib in combination with cytotoxic chemotherapy, specifically carboplatin. Another active area of focus is combining olaparib with immunotherapy agents including atezolizumab (Tecentriq), durvalumab (Imfinzi), and tremelimumab (ClinicalTrials.gov, 2019). The MEDIOLA trial, presented as an abstract at the 2017 San Antonio Breast Cancer Symposium, looked at the combination of olaparib and durvalumab in gBRCAm, HER2-negative metastatic breast cancer. The primary endpoint was disease control rate (DCR). The observed DCR at 12 weeks was 80%. Additional results are pending at this time (Domcheck et al., 2017).
Several other PARP inhibitors have completed clinical trials or clinical trials are underway to determine their place in treatment of breast cancer. The EMBRACA trial was recently published in The New England Journal of Medicine and evaluated the safety and efficacy of talazoparib (Talzenna) compared to standard chemotherapy of physician’s choice (Litton et al., 2018). Standard single-agent chemotherapy options included capecitabine, eribulin, gemcitabine, or vinorelbine in continuous 21-day cycles. Talazoparib, a potent inhibitor of PARP, had a 100 times greater PARP-trapping potential than other PARP inhibitors in preclinical studies. Median PFS was longer in the talazoparib group (8.6 months vs. 5.6 months; hazard ratio, 0.54). Importantly, this trial showed a benefit in patients with a history of central nervous sytem metastases, a subtype with particularly adverse outcomes. Anemia was the most common grade ≥ 3 AE and was reported more frequently in patients receiving talazoparib (55% vs. 38%). Patient-reported outcomes favored treatment with talazoparib (Litton et al., 2018). In October 2018, the FDA approved talazoparib to be used in gBRCAm breast cancer patients with HER2-negative locally advanced or metastatic breast cancer (FDA, 2018). The NCCN Guidelines also updated talazoparib as a Category 1 recommendation for these patients (NCCN, 2018).
SUMMARY
Olaparib was recently FDA approved for the treatment of gBRCAm, HER2-negative metastatic breast cancer in patients who have previously received treatment. This is the first new class of medications to show benefit in metastatic TNBC since the introduction of cytotoxic chemotherapy. In addition, olaparib is only the second oral therapy option for these patients, carrying a better toxicity profile than capecitabine. PARP inhibitors are generally well tolerated, with the most common adverse events being hematologic and gastrointestinal. Although subgroup analyses look promising, more studies will be required to determine the full benefit of olaparib in patients with TNBC.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.American Cancer Society. . Cancer Facts & Figures 2019. 2019 Retrieved from https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html.
- 2.AstraZeneca Pharmaceuticals LP. Lynparza (olaparib) tablets package insert. 2018 Retrieved from https://www.azpicentral.com/lynparza_tb/pi_lynparza_tb.pdf#page=1.
- 3.Ballinger T J, Meier J B, Jansen V M. Current landscape of targeted therapies for hormone-receptor positive, HER2-negative metastatic breast cancer. Frontiers in Oncology. 2018;8:308. doi: 10.3389/fonc.2018.00308. Retrieved from . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balmaña J, Díez O, Castiglione M. BRCA in breast cancer: ESMO clinical recommendations. Annals of oncology : official journal of the European Society for Medical Oncology. 2009;20 Suppl 4:19–20. doi: 10.1093/annonc/mdp116. [DOI] [PubMed] [Google Scholar]
- 5.ClinicalTrials.gov. ClinicalTrials.gov. 2019 Retrieved from https://www.clinicaltrials.gov.
- 6.Dent R A, Lindeman G J, Clemons M, Wildiers H, Chan A, McCarthy N, Carmichael J. Phase I trial of the oral PARP inhibitor olaparib in combination with paclitaxel for first- or second-line treatment of patients with metastatic triple-negative breast cancer. Breast Cancer Research. 2013;15(5):R88. doi: 10.1186/bcr3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domchek S M, Postel-Vinay S, Bang Y-J, Park Y H, Alexandre J, Delord J-P, Kaufman B. An open-label, multitumor, phase II basket study of olaparib and durvalumab (MEDIOLA): Results in germline BRCA-mutated (gBRCAm) HER2-negative metastatic breast cancer (MBC) [Abstract PD6-11]. Cancer Research (2017 San Antonio Breast Cancer Symposium) 2017;78(4 suppl) Retrieved from https://doi.org/ 10.1158/1538-7445.SABCS17-PD6-11. [Google Scholar]
- 8.Dziadkowiec Karolina N, Gąsiorowska Emilia, Nowak-Markwitz Ewa, Jankowska Anna. PARP inhibitors: review of mechanisms of action and BRCA1/2 mutation targeting. Przeglad menopauzalny = Menopause review. 2016;15:215–219. doi: 10.5114/pm.2016.65667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong Peter C, Boss David S, Yap Timothy A, Tutt Andrew, Wu Peijun, Mergui-Roelvink Marja, Mortimer Peter, Swaisland Helen, Lau Alan, O’Connor Mark J, Ashworth Alan, Carmichael James, Kaye Stan B, Schellens Jan H M, de Bono Johann S. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. The New England journal of medicine. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 10.Godet I, Gilkes D M. BRCA1 and BRCA2 mutations and treatment strategies for breast cancer. Integrative Cancer Science and Therapeutics. 2017);4(1):1–7. doi: 10.15761/ICST.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guney Eskiler Gamze, Cecener Gulsah, Egeli Unal, Tunca Berrin. Triple negative breast cancer: new therapeutic approaches and BRCA status. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2018;126:371–379. doi: 10.1111/apm.12836. [DOI] [PubMed] [Google Scholar]
- 12.Litton Jennifer K, Rugo Hope S, Ettl Johannes, Hurvitz Sara A, Gonçalves Anthony, Lee Kyung-Hun, Fehrenbacher Louis, Yerushalmi Rinat, Mina Lida A, Martin Miguel, Roché Henri, Im Young-Hyuck, Quek Ruben G W, Markova Denka, Tudor Iulia C, Hannah Alison L, Eiermann Wolfgang, Blum Joanne L. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. The New England journal of medicine. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Cancer Institute. BRCA mutations: Cancer risk & genetic testing. 2018 Retrieved from https://www.cancer.gov/about-cancer/causes-prevention/genetics/brca-fact-sheet#r2.
- 14.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast cancer. v3.2018. 2018 Retrieved from https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 15.Robson Mark, Im Seock-Ah, Senkus Elżbieta, Xu Binghe, Domchek Susan M, Masuda Norikazu, Delaloge Suzette, Li Wei, Tung Nadine, Armstrong Anne, Wu Wenting, Goessl Carsten, Runswick Sarah, Conte Pierfranco. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. The New England journal of medicine. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 16.Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Female breast cancer. . 2018 Retrieved from https://seer.cancer.gov/statfacts/html/breast.html.
- 17.Tutt Andrew, Robson Mark, Garber Judy E, Domchek Susan M, Audeh M William, Weitzel Jeffrey N, Friedlander Michael, Arun Banu, Loman Niklas, Schmutzler Rita K, Wardley Andrew, Mitchell Gillian, Earl Helena, Wickens Mark, Carmichael James. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet (London, England) 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 18.U.S Food and Drug Administration. FDA approves olaparib for germline BRCA-mutated metastatic breast cancer. 2018 Retrieved from https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm592357.htm.
- 19.U.S. Preventive Services Task Force. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer in Women: Clinical Summary of USPSTF Recommendation. AHRQ Publication No. 12-05164-EF-3. 2013 Retrieved from http://www.uspreventiveservicestaskforce.org/uspstf12/brcatest/brcatestsumm.htm.