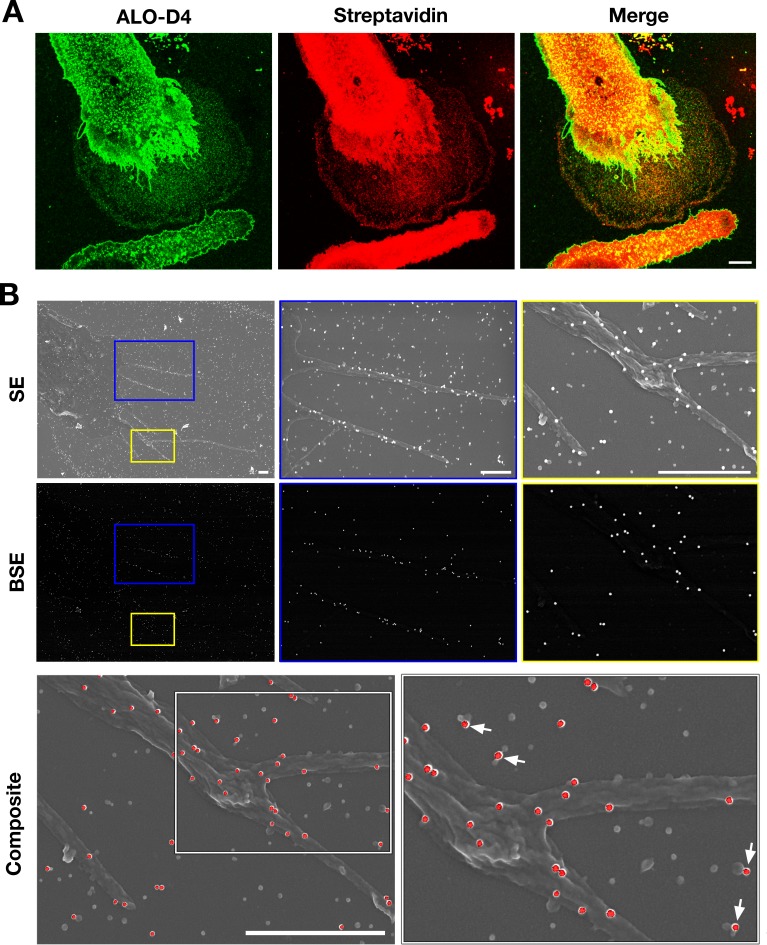
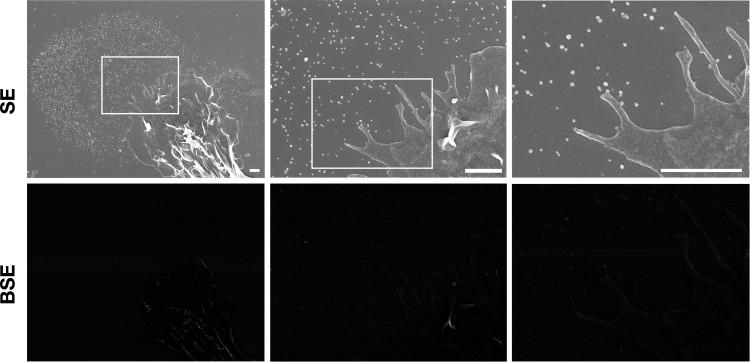
Figure 4. Particles released from the plasma membrane of biotinylated mouse peritoneal macrophages can be detected with streptavidin and ALO-D4 (a cytolysin that binds to the accessible pool of cholesterol).
(A) Biotinylated macrophage particles can be detected with fluorescently labeled streptavidin as judged by confocal microscopy. Macrophages in suspension were biotinylated with Sulfo-NHS-SS-biotin. After plating the cells onto glass coverslips and incubating the cells in macrophage growth medium containing 10% FBS, the cells were washed and then incubated with Atto 647N–labeled streptavidin (red) and Alexa Fluor 488–labeled [15N]ALO-D4 (green). Cells were then fixed with 3% PFA and imaged by super-resolution stimulated emission depletion (STED) microscopy. Streptavidin and ALO-D4 bound to the macrophages as well as the lawn of particles on the surrounding substrate. Scale bar, 5 µm. (B) Biotinylated macrophage particles can be detected with streptavidin-conjugated gold nanoparticles, as judged by scanning electron microscopy (SEM). After biotinylating the plasma membrane of mouse peritoneal macrophages with Sulfo-NHS-SS-biotin, cells were plated onto glass-bottom Petri dishes. On the following day, the cells were incubated with streptavidin-conjugated 40-nm gold nanoparticles. Cells were then fixed with 1% glutaraldehyde and processed for SEM. Secondary electron (SE) and backscattered electron (BSE) images revealed gold nanoparticles on the macrophage cell body, filopodia, and on macrophage particles that had been released onto the substrate. Higher magnification images of the blue and yellow boxes are shown on the right. Composite images (gray, SE; red, BSE) images show colocalization of gold nanoparticles with the particles on the substrate. Higher magnification image of the white box is shown on the right. White arrows point to gold nanoparticles binding to macrophage particles. Three independent experiments were performed; representative images are shown. Scale bar, 1 µm.