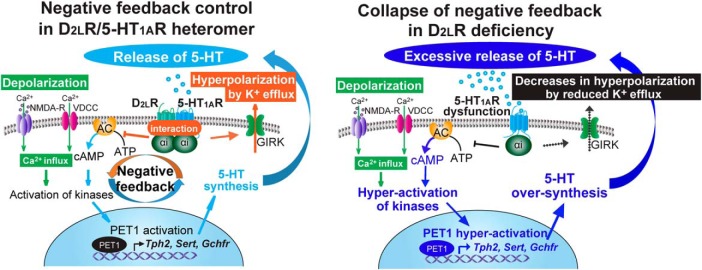
Figure 7.
D2LR deficiency causes collapse of the negative feedback control of the D2LR/5-HT1AR inhibitory G-protein-coupled heteromer in serotonergic neurons. (1) Stress-induced depolarization triggers Ca2+ influx through L-type voltage-dependent calcium channels (L-VDCC) and NMDA receptors (NMDA-R). (2) The Ca2+ influx leads to activation of numerous kinase pathways, resulting in activation of a transcription factor PET1, which is a key modulator of 5-HT synthesis. (3) PET1 activation increases Tph2, Sert, and Gchfr transcript levels, increasing 5-HT synthesis and extracellular release. (4) Extracellular 5-HT activates the D2LR/5-HT1AR heteromer, and induces Gαi activation, inhibition of adenylyl cyclase (AC), and activation of GIRK currents. This results in decreased firing rate of serotonergic neurons and limits 5-HT release. In D2LR deficiency, 5-HT1AR cannot properly function against stress because of the collapse of the negative feedback mechanism, thereby eliciting excessive 5-HT release and stress vulnerability.