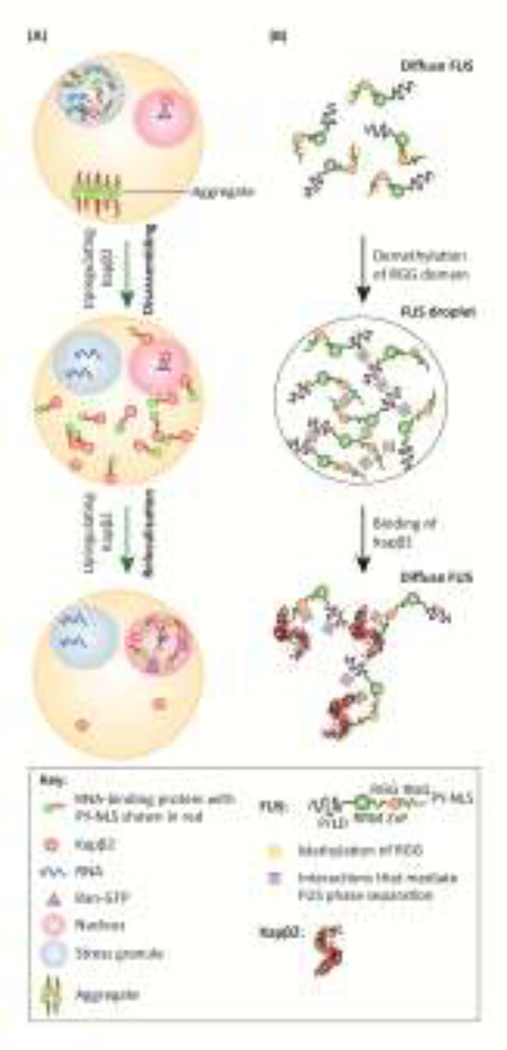
Figure 4. New NIR functions in preventing and reversing aberrant phase separation.
(A) In ALS/FTD, mislocalized nuclear RBPs with PrLDs are recruited into SGs upon stress. Prolonged stress leads to an aberrant phase transition and formation of pathological fibrils (aggregate). Upregulating Kapβ2 reverses recruitment of RBPs into SGs without disassembling the SGs. Upregulating Kapβ2 also solubilizes aggregated RBPs. Once solubilized, Kapβ2 transports RBPs back to the nucleus where Ran-GTP dissociates Kapβ2:RBP complexes enabling RBPs to perform nuclear functions and Kapβ2 to be recycled to catalyze further rounds of disaggregation.
(B) Phase separation of FUS is mediated by the intrinsically disordered PrLDs, as well as cation-π and π-π interactions between tyrosines in the PrLD and arginines in RGG domains. These interactions are modulated by post-translational arginine methylation, where methylation of RGG domains inhibits FUS LLPS. Kapβ2 inhibits FUS LLPS by binding tightly to the PY-NLS and by weakly binding to multiple regions across FUS thereby disrupting FUS-FUS interactions. Direct binding of Kapβ2 to arginines in RGG domains contributes to its chaperoning mechanism by disrupting cation-π and π-π interactions.