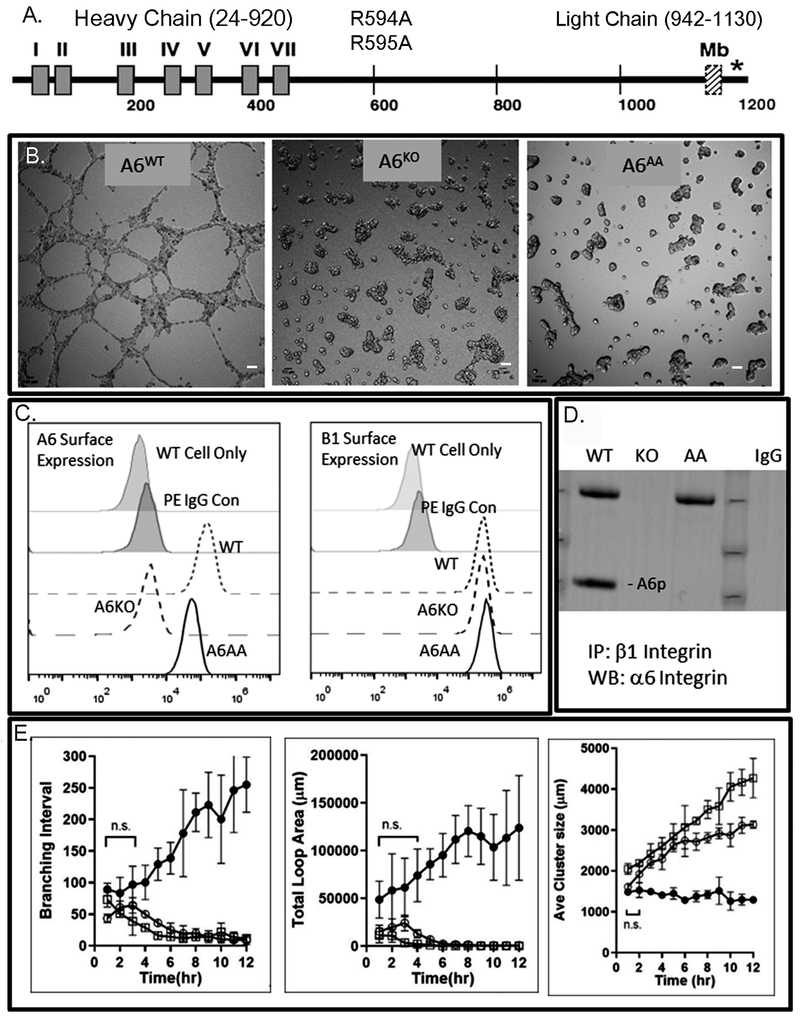
Figure 2.
Gene editing of α6 integrin prevents invasive networks and results in cohesive clusters. A. Schematic showing the amino terminal region of α6 integrin containing the extracellular repeated domains (I-VII) and the position of the R594A and R595A substitution mutations in the α6 integrin heavy chain (amino acids 24-920) and the domains of the light chain (amino acids 942-1130) containing the membrane spanning domain (Mb, striped box) and the cytoplasmic domain (*) at the carboxy terminus. B. DU145 α6WT (left), DU145 α6KO (center) and DU145 α6AA (right) networks at 12 hours on laminin containing Matrigel. Scale bar: 500 microns. C. Flow cytometry profiles of α6 and β1 surface expression in all three cell lines. D. Immunoprecipitation (IP) of β1 integrin, followed by western blot (WB) detection of α6 and α6p (A6p) integrin. E. Network formation on laminin containing Matrigel during 12 hours of incubation using video microscopy. Networks (branching interval, total loop areas and cluster size) were measured using Image J software. Statistical significance was achieved between DU145 α6WT and DU145 α6KO cells and DU145 α6WT and DU145 α6AA cells at all time points unless otherwise indicated as not significant (n.s.) using an unpaired two-tailed student’s t test where p value is < 0.05. Results are representative of at least 3 biological and technical replicates, n=12.