Abstract
The main risk factor for esophageal dysplasia and adenocarcinoma (DAC) is Barrett’s esophagus (BE), characterized by intestinal metaplasia. The critical genomic mechanisms that lead to progression of non-dysplastic BE to DAC remain poorly understood and require analyses of longitudinal patient cohorts and high-resolution assays. We tested BE tissues from 74 patients, including 42 non-progressors from two separate groups of 21 patients each, and 32 progressors (16 in a longitudinal cohort before DAC/pre-progression-BE, and 16 with temporally concurrent but spatially separate DAC/concurrent-BE). We interrogated genome-wide somatic copy number alterations (SCNAs) at the exon level with high-resolution SNP arrays in DNA from formalin-fixed samples histologically confirmed as non-dysplastic BE. The most frequent abnormalities were SCNAs involving FHIT exon 5, CDKN2A/B, or both in 88% longitudinal BE progressors to DAC vs. 24% in both non-progressor groups (P=0.0004). Deletions in other genomic regions were found in 56% of pre-progression-BE but only in 1 non-progressor-BE (P=0.0004). SCNAs involving FHIT exon 5 and CDKN2A/B were also frequently detected in BE temporally concurrent with DAC. TP53 losses were detected in concurrent-BE but not earlier in pre-progression-BE tissues of patients who developed DAC. CDKN2A/p16 immunohistochemistry showed significant loss of expression in BE of progressors vs. non-progressors, supporting the genomic data.
Our data suggest a role for CDKN2A/B and FHIT in early progression of BE to dysplasia and adenocarcinoma that warrants future mechanistic research. Alterations in CDKN2A/B and FHIT by high-resolution assays may serve as biomarkers of increased risk of progression to DAC when detected in BE tissues.
Background
Esophageal adenocarcinoma (EAC) has increased greater than 10-fold over recent decades1–5. Unfortunately, the overall 5-year survival of patients with EAC is below 19%6, with most tumors discovered in advanced stages, after local invasion and/or metastasis occurred2, 3. Most EACs arise in patients with Barrett’s esophagus (BE)7, which is characterized by replacement of the normal squamous esophageal mucosa by columnar epithelium with intestinal metaplasia (IM) and may subsequently progress to dysplasia and adenocarcinoma (DAC), through progressive development of low-grade dysplasia (LGD), high-grade dysplasia (HGD), intramucosal adenocarcinoma and advanced adenocarcinoma8.
Established factors for increased risk of BE and EAC include older age, male sex, white race, gastro-esophageal reflux, smoking, and obesity, whereas gastric H. pylori infection is associated with a decreased risk9. Overall, the presence of BE is associated with a 10–40 fold increased risk for the development of EAC10, 11; however, the overall rate of progression from non-dysplastic BE to EAC is only 0.1–0.5% per patient year, and recent analyses showed a pooled annual incidence of EAC in BE patients of 0.33%10–13. Reported rates of progression from LGD to HGD/EAC are variable, ranging from 0.6% to 13.4% per year14. When HGD develops in BE, the risk for development of EAC greatly increases to 6%−19% per patient-year15. Guidelines for prevention of EAC recommend repeat surveillance endoscopies with multiple 4-quadrant biopsies every 1 to 2 cm along the length of the Barrett’s mucosa, followed by pathological examination to detect BE and dysplasia8, 16. Most patients on surveillance have Barrett’s IM negative for dysplasia, described as BE throughout our study. Since only a small number of patients in the large BE population undergoing endoscopic surveillance will develop dysplasia and/or EAC, understanding the molecular mechanisms of progression and testing for molecular biomarkers that identify BE tissues that are likely to progress to EAC are warranted.
In recent years, a number of studies reported a spectrum of genomic alterations of BE, pre-cancer dysplastic lesions, and EAC17–21. However, these studies have been primarily cross-sectional with limited data reported from longitudinal cohorts22. The difficulty in obtaining large longitudinal cohorts of BE progressors with non-dysplastic BE tissues available for testing before dysplasia/EAC development is well known to the Barrett’s research community. Therefore, there is limited knowledge of genomic alterations in pre-progression-BE histologically confirmed negative for dysplasia, which could help identify patients at early stages of progression to dysplasia and later to EAC, requiring additional data from longitudinal studies. Recent progress in genome-wide techniques to efficiently and accurately test formalin-fixed paraffin-embedded (FFPE) tissues makes it possible to test biopsy fragments that are histologically well-characterized to exclude the diagnosis of dysplasia/EAC before genomic assays are performed17, 23.
In this study we characterized the landscape of somatic copy number alterations (SCNAs) in BE patients, including a longitudinal cohort of BE patients undergoing endoscopic surveillance with a diagnosis of intestinal metaplasia negative for dysplasia, who went on to develop dysplasia or EAC (BE-progressors), compared to a longitudinal cohort who did not develop dysplasia or EAC during the follow-up period (BE-non-progressors).
The group of BE-progressors included BE patients who progressed to low or high-grade dysplasia or invasive adenocarcinoma because current guidelines recommend potential similar clinical management for high and low-grade dysplasia8. Specifically, patients with BE and confirmed HGD should be managed with endoscopic therapy unless they have life-limiting comorbidity and for patients with confirmed LGD and without life-limiting comorbidity, endoscopic therapy is considered the preferred treatment modality, although endoscopic surveillance is an acceptable alternative8. We utilized clinical FFPE tissue samples and used high-definition and high-sensitivity detection single nucleotide polymorphism (SNP) arrays for identification of early genomic alterations that may underlie the molecular mechanisms of BE progression to DAC.
Importantly, the focus of our study on histologically confirmed non-dysplastic BE tissue allowed us to identify genomic lesions involved in the early progression of BE to dysplasia and subsequently to cancer.
Materials and Methods
Patients and Tissue Samples
Samples from seventy-four patients were included in the study, divided into three groups described below (Figure 1 and Supplemental Tables S1 and S2). Patient samples were obtained from archived FFPE samples obtained either during endoscopic biopsy surveillance (all group A and group B samples) or from biopsy, endoscopic mucosal resection or surgical resection esophageal specimens for group C samples. Hematoxylin and eosin (H&E) stained sections of the selected tissue blocks were assessed for the presence, extent, and grade of lesions by two gastrointestinal pathologists (A.R.S. and S.K.) and diagnoses were established with consensus.
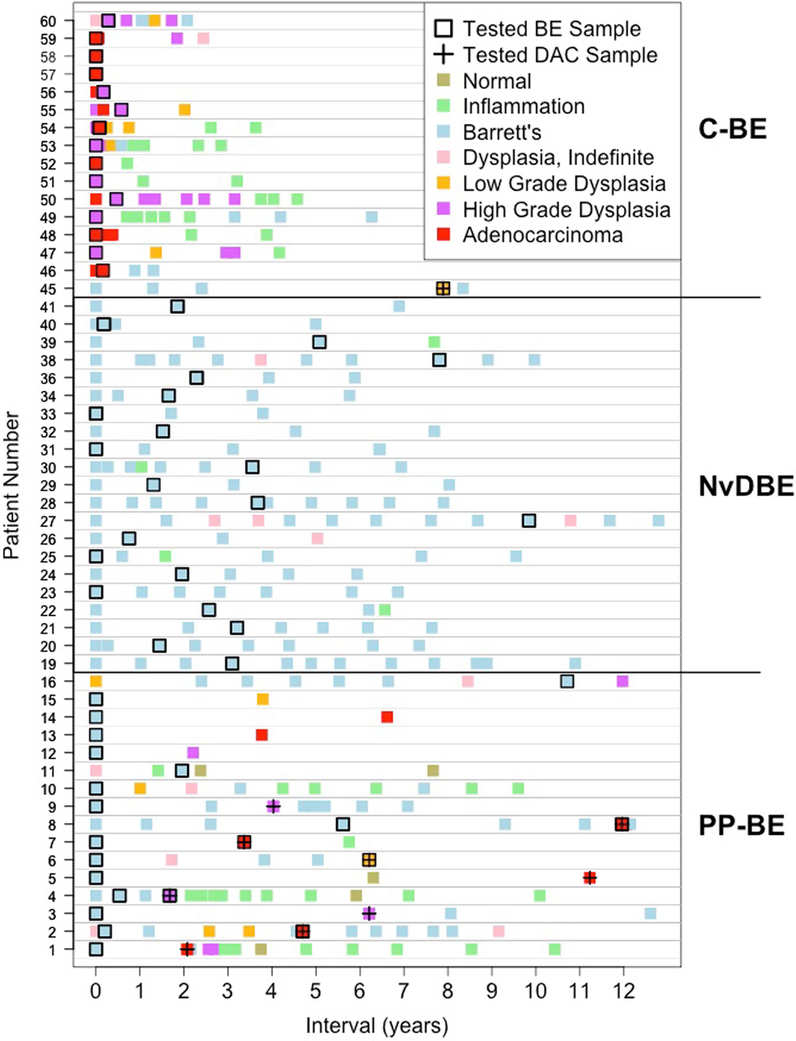
Figure 1. Temporal sampling of tissue specimens for each patient group.
The x-axis shows the interval in years relative to the first available sample. Patients are represented by rows and are grouped as patients who had pre-progression-BE (PP-BE) samples tested, never dysplastic Barrett’s esophagus cohort 1 (NvDBE) patients, and patients from whom the concurrent-BE (C-BE) and/or dysplasia or EAC (DAC) were tested. Each sample is represented by a rectangle with the color indicating the worst lesion in the sample (see legend). Samples tested by Oncoscan SNP-arrays are represented by a black border (BE samples) and/or a cross (dysplasia/EAC samples).
Group A - Longitudinal cohort of BE progressors to dysplasia or EAC:
Sixteen progressor patients were defined by histologic diagnosis of esophageal biopsies as intestinal metaplasia negative for dysplasia in biopsy specimens obtained before development of dysplasia or adenocarcinoma (pre-progression-BE, PP-BE). In this group, progression to dysplasia/EAC occurred within a minimum of one year after the PP-BE sample was collected (median 43 months, range 12–134, standard error of the mean [SEM] = 8), Supplemental Table S1. Group A includes 12 male and 4 female patients, with a mean age of 63 years (range 43–77, SEM = 2) at the time of the first tested sample. The median length of Barrett’s was 5.0 cm (range 1–13, SEM = 0.8). Five patients progressed to high-grade dysplasia (HGD), 4 to low-grade dysplasia (LGD) and 7 to adenocarcinoma (EAC).
Group B - Longitudinal cohorts of BE non-progressors to dysplasia or EAC:
In this study, never dysplastic BE (NvDBE) patients are defined by a histological diagnosis of intestinal metaplasia, negative for dysplasia, in at least two samples taken with an interval of 2 years or greater and by the absence of a diagnosis of dysplasia (HGD or LGD) or EAC in any esophageal biopsy or resection.
We tested two groups of NvDBE patients. The initial group (NvDBE1) was selected from the Columbia Barrett’s Pathology and Archival Database (see Supplemental Table S2) by matching age, gender, and length of BE to the progressors (group A). Second, we tested a separate cohort of NvDBE patients (NvDBE2) by identifying temporally consecutive patients from our database that matched our definition of NvDBE patients and had a total follow-up of at least 5 years. In the NvDBE2 cohort, the median follow-up from the tested sample to the last available sample was 51 months (range 26–114, SEM=5) for NvDBE1 and 85 months (range 59–146, SEM=5) for NvDBE2. There were no statistically significant differences between the two NvDBE groups or between the NvDBE groups and the pre-progression BE patients in age, gender, BE length, percent of IM, and sample type (see Supplemental Table S1).
Group C - Progressors with concurrent BE (C-BE):
This group includes twenty-one patients with dysplasia or EAC who had temporally concurrent but spatially separate intestinal metaplasia samples from the same procedure (endoscopic biopsy, EMR, or surgical resection). The C-BE group includes 16 patients who did not have baseline pre-progression biopsies available and 5 patients from the PP-BE group A who had baseline pre-progression samples as well as concurrent BE samples available. In this group, the esophageal mucosa with intestinal metaplasia, negative for dysplasia, was tested and described as concurrent-BE (Supplemental Table S1).
Additionally, we tested samples of histologically confirmed dysplasia or adenocarcinoma from 10 patients from the PP-BE and C-BE groups who had BE samples tested.
Most patients were identified in the Columbia Barrett’s Pathology and Archival Database, which includes 32,725 patients with FFPE esophageal specimens from January 2000 to 2017 (See Supplemental Table S2). Two pre-progression-BE cases were submitted from the University of Pennsylvania and two were from the University of Pittsburgh. The study was approved by the Institutional Review Board of Columbia University.
Tissue Processing
One specimen of intestinal metaplasia negative for dysplasia was tested for each patient, from FFPE-embedded tissue blocks. For ten progressors, tissues samples of dysplasia or adenocarcinoma were also tested. Each selected tissue block from endoscopic procedures generally included multiple biopsy fragments containing IM, dysplasia or EAC. For samples from resection specimens, spatially separate representative areas of IM, dysplasia, or EAC were macrodissected from tissue sections. To ensure adequate amounts of tissue for DNA extraction, the entire tissue was scrapped from small biopsy samples, whereas for larger biopsies separate fragments of squamous epithelium or cardio-oxyntic mucosa not containing IM were excluded.
Lesional areas for macrodissection were marked on guiding H&E slides. The IM samples used for DNA extraction had areas of intestinal metaplasia ranging from 1 to 90% (median = 20%, SEM = 8%) of the overall mucosa in pre-progression-BE, 5 to 80% in NvDBE1 samples (median = 20%, SEM = 5%), 5 to 80% in NvDBE2 samples (median = 30%, SEM = 5%), and 10 to 90% in concurrent-BE samples (median = 30%, SEM = 6%), as estimated in the guiding H&E-stained sections. There were no significant differences in the area of IM between groups (Supplemental Table S1).
DNA Extraction and Quantification
For DNA extraction, we used 7 micron thick unstained tissue sections. Genomic DNA was isolated using the QIAamp DNA FFPE Tissue Kit (Qiagen, Germantown, MD) following the manufacturer’s recommendations. DNA was quantified by fluorimetry using the Quant-iT dsDNA HS Assay (Invitrogen, Carlsbad, CA) and measured by the Qubit fluorimeter (Invitrogen, Carlsbad, CA).
OncoScan SNP Arrays
The DNA samples were processed for identification of SCNAs using the OncoScan FFPE Assay or the OncoScan CNV Assay (Affymetrix, Santa Clara, CA); both utilize the Molecular Inversion Probe (MIP) assay technology to interrogate genome-wide SNPs for allelic frequency and copy number alterations23, 24. Annealing, amplification and labeling were performed as described in the OncoScan FFPE Assay Manual Rev.1 (Affymetrix). Briefly, 79 ng of DNA from each sample were annealed with the MIP solution for 18 hours followed by gap filling with AT/GC nucleotides. After removing the unligated, non-gap-filled, linear probes through exonuclease treatment, a cleavage enzyme was added to linearize the gap-filled circular MIP followed by amplification and enrichment of the gap-filled, linearized MIP through 1st and 2nd PCR. After fragmentation with HaeIII enzyme, the enriched PCR product was hybridized to the GeneChip Oncoscan Array (Affymetrix) or GeneChip Oncoscan CNV Array (Affymetrix) for 16–18 hours at 49°C and 60 rpm rotation. After hybridization, the arrays were washed, stained using GeneChip Fluidics Station 450 (Affymetrix) and scanned using GeneChip Scanner 3000 7G (Affymetrix). The CEL files generated after scanning were converted to OSCHP files and analyzed using Chromosome Analysis Suite 3.2 (ChAS) Software (Affymetrix).
The B-allelic frequency (BAF) and log 2-ratio plots were visually inspected using ChAS and segments were validated and manually adjusted. In addition, an automated algorithm using R statistical software25 counted the number of consecutive probes on the 5’ and 3’ regions around FHIT exon 5 with weighted log2 ratios less than zero. FHIT exon 5 was considered deleted if there were five of more consecutive probes with weighted log2-ratios less than zero on each side of exon 5 and the minimum weighted log2-ratio of those probes were less than −0.5 on both sides of exon 5.
Immunohistochemistry
FFPE tissue sections, five micron thick, were used for immunohistochemical staining with pre-diluted, ready to use anti-p16 mouse monoclonal antibody clone E6H4 (Ventana Medical Systems, Inc., Tucson, AZ) in the automated BenchMark ULTRA instrument (Ventana Medical Systems). Labeling with p16 antibody was detected with ultraView Universal DAB Detection Kit (Ventana Medical Systems) as per the manufacturer protocol. All sections were counterstained with Gill’s hematoxylin and ammonium hydroxide bluing solution.
The immunohistochemistry-stained slides were digitally scanned at 200× resolution in a SCN 400 scanner (Leica Biosystems Inc., Buffalo Grove, IL) and p16 expression was scored based on the maximal intensity of each sample for both nuclear or cytoplasmic staining using the following scale: 0 (no expression), 1 (brownish blue), 2 (light brown) and 3 (dark brown).
Statistical Analyses
Fisher’s exact test was used to identify differences in the frequency of genomic alterations between groups (e.g. pre-progression-BE vs. NvDBE). Correction for multiple testing was performed using the Benjamini & Hochberg false discovery rate method. Confidence intervals were calculated using the exact binomial method. The Mann-Whitney rank sum test with continuity correction was used to assess differences in age, length of BE, area of lesional tissue, and p16 expression. Two-tailed t-tests were used to determine whether there were significant differences between groups in size of non-diploid genome. R Statistical Software25 was used for all statistical calculations. P values <0.05 were considered significant.
Results
Somatic genomic copy number alterations in pre-progression non-dysplastic Barrett’s intestinal metaplasia are highly prevalent in patients who progress to dysplasia and adenocarcinoma
Most BE patients who are on endoscopic surveillance have intestinal metaplasia, negative for dysplasia, and only a small subset of these patients will progress to dysplasia or EAC. Genomic characterization, beyond histopathology, of BE samples that are negative for dysplasia may be useful to separate progressors from non-progressors to better understand the biology underlying progression from pre-neoplastic IM lesions to dysplasia and adenocarcinoma and to potentially help tailor endoscopic surveillance and therapy. Therefore, we identified a group of patients with FFPE endoscopic biopsies obtained before the development of dysplasia or EAC (described as pre-progression-BE), and compared them with two separate groups of BE patients who did not progress to dysplasia or EAC (NvDBE1 and NvDBE2 patients). Both non-progressor and progressor groups represent longitudinal cohorts. See Figure 1 for representation of progression and longitudinal sampling of the patient groups.
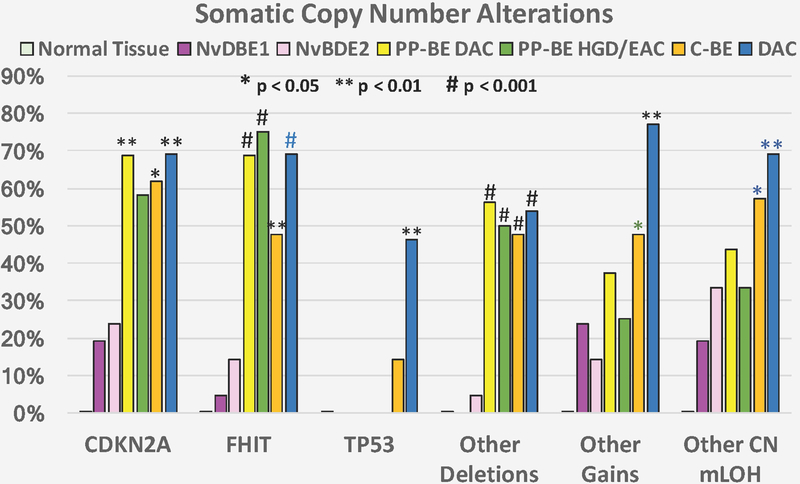
Fifty-eight patients had SNP-array results in the longitudinal cohort of BE patients, including 21 NvDBE1 and 21 NvDBE2 non-progressors and 16 pre-progression-BE from progressors (Tables 1 and 2, and Figures 1 and S1). Six patients with pre-progression-BE also had non-esophageal normal tissues tested as controls, which showed no SCNAs. The most frequent SCNAs in pre-progression-BE affected genomic regions that include the FHIT and CDKN2A genes. Deletion of FHIT, including exon 5, was found in 11/16 (69%) of pre-progression-BE cases but only in 1/21 (5%) of NvDBE1 (P=0.0004, Table 1) and 3/21 NvDBE2 (P=0.0032). The size of the deleted segments of FHIT involving exon 5 in the pre-progression-BE samples ranged from 195 to 614 kb (Table 2 and Figure S2).
Table 1.
Summary data of SNP arrays for each group of samples. NvDBE1: BE from never dysplastic Barrett’s esophagus cohort 1; NvDBE2: BE from never dysplastic Barrett’s esophagus cohort 2; PP-BE LGD/HGD/EAC: pre-progression-BE from patients who subsequently (over one year later) progressed to dysplasia (LGD or HGD) or EAC; PP-BE HGD/EAC: pre-progression-BE from patients who subsequently (over one year later) progressed to HGD or EAC; C-BE: BE from temporally concurrent but spatially separate esophageal areas of patients with dysplasia or EAC; this group includes 5 samples from 5 patients who also had baseline PP-BE samples tested and 16 patients who did not have PP-BE samples available; DAC: samples from dysplasia or EAC; this group includes 10 patients from the PP-BE and C-BE groups who had BE samples tested. In each of the groups listed in each column, there was one sample tested per patient. Individual cells in the table contain the number of samples with each type of somatic copy number alteration (SCNA) listed on the left, the percent of positive samples in each group (in round parenthesis), and the Fisher’s exact test P-value in comparison to NvDBE1 (first square parentheses in each cell) and NvDBE2 (second square parentheses). Statistically significant SCNAs (P<0.05) are highlighted in bold.
| SCNA | Normal Tissue | NvDBE1 | NvDBE2 | PP-BE LGD/HGD/EAC | PP-BE HGD/EAC | C-BE | DAC |
|---|---|---|---|---|---|---|---|
| Number of cases | 6 | 21 | 21 | 16 | 12 | 21 | 10 |
| FHIT Deletion Exon 5 | 0 (0%) | 1 (5%) | 3 (14%) |
11 (69%) [0.0004] [0.0032] |
9 (75%) [0.0007] [0.0037] |
10 (48%) [0.0096] [0.057] |
8 (80%) [0.0007] [0.0030] |
| CDKN2A/2B | 0 (0%) | 4 (19%) | 5 (24%) |
11 (69%) [0.0094] [0.014] |
7 (58%) [0.083] [0.11] |
13 (62%) [0.018] [0.045] |
6 (60%) [0.04] [0.12] |
| Homozygous deletion or deletion in area of complete LOH | 0 (0%) | 2 (10%) | 1 (5%) | 5 (31%) [0.28] [0.12] |
5 (42%) [0.13] [0.035] |
5 (24%) [0.45] [0.25] |
1 (10%) [1.0] [1.0] |
| Hemizygous deletion | 0 (0%) | 0 (0%) | 2 (10%) | 4 (25%) [0.051] [0.51] |
2 (17%) [0.19] [0.78] |
6 (29%) [0.039] [0.26] |
2 (20%) [0.12] [0.58] |
| Copy neutral partial LOH | 0 (0%) | 2 (10%) | 2 (10%) | 2 (13%) [1.0] [1.0] |
0 (0%) [0.64] [0.78] |
2 (10%) [1.0] [1.0] |
3 (30%) [0.3] [0.3] |
| TP53 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) [1.0] [1.0] |
0 (0%) [1.0] [1.0] |
3 (14%) [0.23] [0.23] |
5 (50%) [0.0024] [0.0030] |
| Other Somatic Deletions | 0 (0%) | 0 (0%) | 1 (5%) |
9 (56%) [0.0004] [0.0019] |
6 (50%) [0.0028] [0.0094] |
10 (48%) [0.0020] [0.0096] |
5 (50%) [0.0024] [0.010] |
| Somatic Gains | 0 (0%) | 5 (24%) | 3 (14%) | 6 (38%) [0.55] [0.19] |
3 (25%) [1.0] [0.85] |
10 (48%) [0.23] [0.0096] |
7 (70%) [0.024] [0.0061] |
| Somatic Copy Neutral pLOH | 0 (0%) | 4 (19%) | 7 (33%) | 7 (44%) [0.20] [0.83] |
4 (33%) [0.42] [1.0] |
12 (57%) [0.033] [0.23] |
7 (70%) [0.017] [0.12] |
| Any FHIT or CDKN2A SCNA | 0 (0%) | 5 (24%) | 5 (24%) |
14 (88%) [0.0004] [0.0008] |
10 (83%) [0.0028] [0.0037] |
15 (71%) [0.0096] [0.0096] |
9 (90%) [0.0024] [0.0030] |
| Any FHIT or CDKN2A SCNA or Other Somatic Deletions | 0 (0%) | 5 (24%) | 5 (24%) |
14 (88%) [0.0004] [0.0008] |
10 (83%) [0.0028] [0.0037] |
17 (81%) [0.0020] [0.0041] |
9 (90%) [0.0024] [0.0030] |
Table 2.
Somatic copy number alterations (SCNAs) in pre-progression-BE of progressors, and in BE of non-progressors. Patient numbers are followed by letters L (LGD), H (HGD) or C (carcinoma), indicating the most advanced lesion that the patient progressed to. Representative genes found in smaller SCNA segments and size of SCNA segments in Kb are in parenthesis. * Deletion of 3p14.2 including FHIT exon 5. ND: not detected; Ln: Copy number loss without loss-of-heterozygosity (LOH); Lp: Loss with partial (clonal) LOH; Lc: Loss in segment of complete LOH; Gn: Gain without LOH; Gp: Gain with partial LOH; Gc: Gain in segment of complete LOH; CNp: Copy neutral partial LOH. ^ - Multiple deletions: 1p36.22 Lc (MTOR, 12); 5q22.2 Ln (237); 9p13.3 Lp (1232); 12p13.31 Lc (OVOS, 248); 15q14 Lc (SPRED1, 61); 18q21.1 Lc (SMAD7, 56); 20p12.3 Ln (PLCB1, 178); 22q12.2 Lc (PLA2G3, 202). # Complex interspersed deletions and gains in the 9p genomic region.
| Patient Number | * FHIT Deletion | 9p Changes Including CDKN2A / CDKN2B |
Other Deletions | Gains | Copy Neutral Partial LOH |
|---|---|---|---|---|---|
| Pre-Progression-BE (N=16) | |||||
| 1 C | ND | ND | ND | ND | ND |
| 2 C | (262) | 9p24.3–21.3 Lp (20450) # | 6p11.2 Lp (PRIM2, 543) | 4p15.1 Gp (PCDH7, 1389); 9p22.3–13.2 Gp (16155) # |
ND |
| 3 H | ND | 9p21.3 Lc (8) | ND | ND | 3q26.1 (1529) |
| 4 H | (356) | ND | 20p12.3 Ln (PLCB1, 177) | ND | ND |
| 5 C | (375) | ND | 18q21.2 Lc (DCC, 287) | ND | ND |
| 6 L | ND | 9p21.3 Lp (23) | ND | 10q11.22 Gp (PPYR1, 165) | 9p24.3–13.2 (36919) |
| 7 C | (614) | 9p21.3 Ln (3197) | 9p21.3–21.1 Lp (3762) | 8p23.1 Gp (GATA4, 1283) | ND |
| 8 C | (195) | ND | ND | ND | ND |
| 9 H | ND | ND | ND | ND | 9p21.3 (DMRTA1, 3461) |
| 10 L | (276) | 9p24.3-q34.12 CNp (109573) | 14q22.1 Ln (STYX, 214) | 9q21.33–34.3 Gn (31239) | 9p24.3-q34.12 (109573) |
| 11 L | ND | 9p21.3 Lp (205) | 12p13.31 Ln (OVOS, 193) | 6q24.3 Gp (SAMD5, 319) | ND |
| 12 H | (281) | 9p21.3 Lc (176) | Multiple deletions ^ | ND | 7q21.3 (1989); 9p24.3–13.1 (36541) |
| 13 C | (531) | 9p21.3 Lc (153) | ND | ND | 9p24.3–13.3 (35476) |
| 14 C | (316) | 9p21.3 Ln (493) | 18q21.1 8 Ln (SMAD7, 35) | 3q12.2 Gp (TFG, 137) | ND |
| 15 L | (378) | 9p21.3 CNp (186) | 5q22.2 Lc (APC, 51) | ND | 9p21.3 (186) |
| 16 H | (215) | 9p21.3 Lp (3647) | ND | ND | ND |
|
TOTAL
PP-BE |
11 (69%) | 11 (69%) | 9 (56%) | 6 (38%) | 7 (44%) |
| Never dysplastic BE of Non-Progressors Cohort 1 (N=21) | |||||
| 19 | ND | 9p21.3 Ln (67) | ND | 4q13.1 Gn (LPHN3 198); 17p13.1 Gp (GAS7 411) |
9p24.3–13.3 (35929) |
| 22 | ND | ND | ND | 10q11.22 Gp (PPYR1 273) | ND |
| 23 | ND | 9p21.3 Lc (114) | ND | 5q12.3 Gp (SREK1 813) | ND |
| 26 | ND | 9p24.3–11.2 CNp (54571) | ND | ND | 9p24.3–11.2 (54571) |
| 28 | ND | ND | ND | 5p13.3 Gc (PDZD2 58) | ND |
| 29 | ND | 9p24.3–21.2 CNp (25609) | ND | ND | 9p24.3–21.2 (25609) |
| 30 | ND | ND | ND | 5q35.1 Gc (RANBP17 195); 16p13.3 Gc (PDPK1 103) |
9p24.3–21.1 (19543) |
| 38 | (217) | ND | ND | ND | ND |
| 20, 21, 24, 25, 27, 31–34, 36, 39–41: | |||||
|
TOTAL
NvDBE 1 |
1 (5%) | 4 (19%) | 0 (0%) | 5 (24%) | 4 (19%) |
| Never dysplastic BE of Non-Progressors Cohort 2 (N=21) | |||||
| 65 | (261) | 9p21.3 CNp (1354) | 9p21.3 (1354) | ||
| 66 | 10q11.22 Gp (PPYR1 743) | ||||
| 72 | 6p25.3 Gp (FOXC1/F2 692) | 9p24.3–13.3 (19009) | |||
| 73 | 9p24.3–21.1 (1577) | ||||
| 74 | 9p21.3 CNp (10629) | 7p21.3-q31.1 Gp (5224) 8p23.1-q24.21 Gp (4608) 10p12.2-q26.11 Gp (7828) |
9p24.3–21.1 (31583) 17q22–25.3 (26913) 18p11.32-q23 (78306) |
||
| 76 | (591) | 9p21.3 Lp (221) | 9p24.3–13.3 (35390) 20q11.21–13.33 (28707) |
||
| 79 | 9p21.2 (198) | ||||
| 81 | 9p21.3 Lc (15) | 9p21.3–21.1 Lp (7834) | 9p24.3–13.1 (30743) | ||
| 82 | (560) | 9p21.3 Lp (406) | |||
| 67–71, 75, 77, 78, 80: | |||||
|
TOTAL
NvDBE2 |
3 (14%) | 5 (24%) | 1 (5%) | 3 (14%) | 7 (33%) |
| NvDBE 1+2 | 4 (10%) | 9 (21%) | 1 (2%) | 8 (19%) | 11 (26%) |
As summarized in Table 1, overall SCNAs in chromosome 9p including the CDKN2A/B gene were found in 11/16 cases (69%) of pre-progression-BE and in 4/21 (19%) NvDBE1 (P=0.0094) and 5/21 (24%) NvDBE2 (P=0.014) patients. Hemizygous deletions of CDKN2A/B with partial (clonal) loss of heterozygosity (pLOH) were present in 25% of pre-progression-BE, 0% of NvDBE1, and 10% of NvDBE2, whereas deletions without pLOH (likely homozygous) were present in 31% of pre-progression-BE compared to 10% of NvDBE1 and 5% of NvDBE2. Copy neutral pLOH (representing clonal somatic uniparental disomy26) involving CDKN2A/B was detected in 13% of pre-progression-BE, 10% of NvDBE1 and 10% of NvDBE2 cases. The size of SCNAs in chromosome 9p ranged from a very focal deletion of 8 kb involving CDKN2A/B to the complete 9p arm (Table 1 and Figure S2). Figure 3 shows a representative BE progressor patient, comparing normal tissue showing no evidence of deletions with pre-progression-BE carrying focal homozygous deletions of FHIT exon 5 and CDKN2A/B more than three years before diagnosis of EAC. The EAC tissue showed the same FHIT deletion as well as extended homozygous deletion of CDKN2A/B, loss of one arm of chromosome 9p, and uniparental somatic duplication of the other arm of 9p.
Figure 3. Genomic alterations of multiple temporally and spatially separate samples for patient 7.
Weighted log2 ratio and B-allele frequency (BAF) plots were obtained with ChAS software. Panel A shows the region of chromosome 9p21.3–21.1 with the vertical interrupted line located over CDKN2A. Panel B shows the region of chromosome 3 including FHIT gene, with the vertical line over exon 5. Four samples from patient 7 are represented, including normal non-esophageal tissue, pre-progression-BE 3 years and 4 months before progression (PP-BE), concurrent-BE (C-BE), and EAC. Panel A plots show deletion of a region of chromosome 9p including CDKN2A in pre-progression-BE (PP-BE), concurrent-BE (C-BE), and EAC. In the pre-progression-BE sample, this region comprises two hemizygous deletions highlighted by the “bubbles” in the BAF plot, and a homozygous deletion of CDKN2A, marked by lower log2 ratio and loss of LOH with recovery of the middle line in the BAF plot due to the presence of normal cells in the sample. The EAC samples shows extension of the homozygous deletion encompassing CDKN2A and somatic copy neutral duplication of an entire 9p arm, as shown by the 4 lines in the BAF plot. The concurrent-BE lesion is similar to that seen in the EAC, but the observed alterations are less prominent, probably due to much lower percentage of affected cells. Panel B plots show homozygous deletion of exon 5 of FHIT in the pre-progression-BE, concurrent-BE and EAC samples, and additionally, copy neutral partial LOH (CNpLOH) of chromosome 3 in EAC. Loss of CNpLOH in the region of FHIT deletion is likely due to the presence of normal cells with intact FHIT in the sample.
Other somatic deletions were found in 56% of pre-progression-BE but not in NvDBE1 patients (P=0.0004) and only 1 (5%) of NvDBE2 patients (P=0.0019), and included hemizygous deletions with pLOH in 6p11.2 and 9p, and deletions without pLOH in 5q22.2, 12p13.31, 14q22.1, 18q21.2, and 20p12.3 (Table 2 and Figure S1). One pre-progression-BE sample (patient 12) had somatic deletions in multiple chromosomes (Table 2). In addition, somatic gains were detected in 38% of pre-progression-BE compared to 24% of NvDBE1 and 14% of NvDBE2, and copy neutral pLOH, involving chromosome 9p and other regions, was seen in 44% of pre-progression-BE, 19% of NvDBE1 and 33% of NvDBE2. Interestingly, no TP53 SCNAs were found in pre-progression-BE or NvDBE patients (Table 1).
When the four progressors to LGD were excluded from the pre-progression-BE cohort, comparable results were observed (Figure 2 and Table 1), suggesting that progression to LGD, HGD or EAC shares similar copy number genetic alterations.
Figure 2. Somatic copy number alterations in samples tested by Oncoscan SNP arrays.
The bars represent the proportion of samples in each group harboring the specific somatic copy number alterations (SCNAs) indicated in the x-axis. NvDBE1: BE tissue of patients who never progressed to dysplasia or EAC cohort 1 (N=21). NvDBE2: BE tissue of patients who never progressed to dysplasia or EAC cohort 2 (N=21). PP-BE: pre-progression biopsy samples of intestinal metaplasia negative for dysplasia at a time point at least one year before dysplasia or EAC biopsy diagnosis in patients who progressed to dysplasia or EAC (N=16). C-BE: Concurrent non-dysplastic intestinal metaplasia spatially separate from co-existing dysplasia or esophageal adenocarcinoma sampled at the same time point, representing a background of intestinal metaplasia in the esophagus of patients with dysplasia or EAC (N=21). DAC: tissue lesion containing dysplasia (low- or high-grade) or EAC from progressor patients (N=10). Normal tissue: 6 patients who were tested for BE also had matched non-esophageal normal tissues tested. Significance for comparisons of each lesion type with NvDBE controls is indicated as follows: * P<0.05, ** P<0.01, # P<0.001 (Fisher’s exact test), in comparison to both NvDBE1 and NvDBE2 (black), NvDBE1 only (blue) or NvDBE2 only (green).
The highest sensitivity for separating pre-progression-BE of progressors from BE of non-progressors was achieved with the combination of FHIT and CDKN2A/B SCNAs. FHIT and/or CDKN2A/B SCNAs were present in 88% of pre-progression-BE compared to 24% of NvDBE1 (P=0.0004) and NvDBE2 (P=0.0008, Table 1). This results in a sensitivity of 88% (95% C.I = 49–90%) and specificity of 76% (95% C.I. = 65–99%) for separating pre-progression-BE of progressors from BE of non-progressors.
Somatic genomic copy number alterations in non-dysplastic concurrent intestinal metaplasia, dysplasia, and adenocarcinoma lesions of BE progressors
Twenty-one cases of concurrent-BE were tested with SNP arrays (Supplemental Table S1). Five of these patients were from the progressor group with pre-progression-BE tested. Sixteen additional concurrent-BE lesions tested were from patients without pre-progression-BE available. Additionally, ten dysplasia/EAC lesions were tested with SNP arrays (Table S1). The tested EAC lesions were all intramucosal adenocarcinomas, except cases 8 (pT1b) and 5 (pT3) (Supplemental Table S4).
As summarized in Table 2 and detailed in Tables S3 and S4, losses of 3p14.2 involving FHIT exon 5 were present in only one NvDBE1 patient (5%) and 3 NvDBE2 patients (14%), and were more frequent in dysplasia/EAC (80%) and concurrent-BE (48%). Likewise, deletions or copy neutral pLOH of chromosome 9p involving CDKN2A/B were present in 19% of NvDBE1 and 24% of NvDBE2 but were more frequent in dysplasia/EAC (60%) and concurrent-BE (62%). Copy number gains were more frequently seen in dysplasia/EAC (70%) and concurrent-BE (48%) than NvDBE1 (24%) or NvDBE2 (14%), Table 1, Figure 2 and Supplemental Figure S1.
In addition to the alterations in 3p14.2 involving FHIT exon 5 and in 9p involving CDKN2A/B, somatic gene losses were detected in 18q21.1–18q21.2 including BCL2 and SMAD7, 17p13.1 (TP53), 11p15.4(ILK) and 19p13.2(MAP2K7) and gains in 8q24.21(MYC) and 6q24.2–6q25.1(SAMD5) in dysplasia/EAC and concurrent-BE but not in NvDBE1 or NvDBE2 (Supplemental Tables S3 and S4).
SCNA alterations in dysplasia or EAC lesions (Supplemental Table S4) were more extensive than in concurrent-BE (Supplemental Table S3). The average fraction of non-diploid genome was low in NvDBE1 (0.01%) and NvDBE2 (0.02%), increased to 0.11% in PP-BE and 2.0% in concurrent-BE, and was highest in dysplasia or EAC (6.1%, P=0.008 vs. NvDBE1 and 0.011 vs. NvDBE2, Supplemental Table S5). The average fraction of non-diploid genome was higher in EAC (10%) than in low and high-grade dysplasias (1.8%).
Reduced p16 expression in Barrett’s intestinal metaplasia of progressors versus non-progressors to dysplasia and esophageal adenocarcinoma
The increased proportion of CDKN2A/B locus copy number alterations in pre-progression-BE vs. control non-progressor BE would predict lower expression of the gene product p16, so we examined samples from the PP-BE and NvDBE1 groups by immunohistochemistry. The results are shown in Figure 4. Normal squamous epithelium, normal oxyntic glands and normal cardia type glands frequently showed heterogeneous positive immunoreactivity for p16 mainly observed in the nucleus. Nuclear p16 expression was significantly higher in IM of non-progressors (mean expression score = 1.32, SEM = 0.08) than in IM of progressors (mean expression scores = 0.32, SEM = 0.04), P=0.023. p16 nuclear expression was lower in IM of progressors with SCNAs involving CDKN2A/B (mean expression score = 0.31, SEM = 0.07) than in IM of progressors without SCNAs involving CDKN2A/B (mean expression score = 1.08, SEM = 0.05), but did not reach statistical significance (P=0.053).
Figure 4. p16 alterations detected by immunohistochemistry (IHC) in BE of progressors and effect of somatic loss of CDKN2A/B locus.
(A) BE sample with no SCNAs in the CDKN2A/B locus from a control never dysplastic, non-progressor patient, shows strong nuclear expression of p16 in a gland with IM (score 3). (B1) pre-progression IM sample from a progressor patient with somatic loss of CDKN2A/B locus, shows absent nuclear expression (score 0), whereas in (B2), the adjacent squamous epithelial cells of the same patient represented in B1 show strong p16 nuclear expression. Arrows: p16 nuclear expression in IM epithelium; Arrowheads: p16 nuclear expression of normal squamous epithelial cells. Original magnification 200×. (C) Average p16 nuclear IHC scores comparing never-dysplastic BE cohort 1 (non-progressor) vs. non-dysplastic BE of progressors (including PP-BE and C-BE lesions). (D) Average p16 nuclear IHC scores in non-dysplastic BE comparing lesions with and without SCNAs involving CDKN2A/B. Error bars represent the standard error of the mean. P values were calculated with the Mann-Whitney rank sum test with continuity correction.
Discussion
The key early molecular alterations that trigger progression of Barrett’s esophagus to dysplasia and esophageal adenocarcinoma remain poorly understood. Progress has been hampered by the limited availability of histologically confirmed non-dysplastic BE tissues temporally preceding dysplasia and EAC. Here we report the genome-wide somatic alterations in pre-progression Barrett’s samples from a longitudinal cohort of patients to determine the earliest SCNAs and potential genes involved in the progression to dysplasia and EAC; we also tested BE concurrent to DAC, the latter providing insight into alterations that likely occur later when dysplasia and cancer develop, and compared genomic alterations with two separate control groups of never dysplastic BE non-progressors. In addition to potential mechanistic implications, genomic alterations detectable in tissue samples obtained during surveillance of Barrett’s esophagus patients may serve as biomarkers for risk stratification to improve detection of progressors to dysplasia and EAC. A number of recent studies reported a spectrum of genomic alterations in non-dysplastic BE and in dysplastic pre-cancer and EAC lesions17–21. However, most studies were cross-sectional17, 18, 20, 21 and there are limited data from pre-progression longitudinal samples reporting genome-wide alterations in BE22, 27. Furthermore, our study is the first to study genome-wide copy number alterations from longitudinal BE samples characterized histologically as negative for dysplasia, which is possible because we performed high-resolution SNP arrays from FFPE, in contrast to previous studies that tested separate fresh tissue samples22, or used a limited 243 gene next generation sequencing (NGS) panel27.
We tested biopsies from three longitudinal cohorts: 1. Progressors to dysplasia or EAC (PP-BE), who had BE samples described as pre-progression-BE obtained before the diagnosis of dysplasia or EAC (median 43 months); 2. never dysplastic BE non-progressors cohort 1 (NvDBE1), who had a diagnosis of BE and did not progress to dysplasia or EAC during a median 51 months of follow-up from the tested biopsy and median 83 months of observation, identified by matching for age, gender and length of BE of the progressor group; and 3. never dysplastic BE non-progressors cohort 2 (NvDBE2), who had a diagnosis of BE and did not progress to dysplasia or EAC during a median 85 months of follow-up from the tested biopsy and median 145 months of observation, identified from temporally consecutive patients in our database.
In contrast to a previous NGS study27, the high-resolution SNP arrays used in our study permit highly sensitive detection of copy number alterations in small genomic regions, at exon resolution, and in small cellular subpopulations in each tested sample. The most frequent SCNAs in non-dysplastic BE involved FHIT exon 5 and/or CDKN2A/B, and their combined alterations were significantly more prevalent in pre-progression-BE (88%) than in both NvDBE cohorts (24%). We detected CDKN2A/B SCNAs in 69% of BE progressors to dysplasia or EAC vs. 19% of NvDBE1 and 24% of NvDBE2 non-progressor BE patients, and gene losses involving FHIT exon 5 were detected in 69% of BE progressors to dysplasia or EAC and only in 5% of NvDBE1 and 14% of NvDBE2 non-progressors, suggesting that SCNAs involving CDKN2A/B, FHIT or both may represent sensitive and specific early biomarkers of future progression to dysplasia and EAC in non-dysplastic BE, that warrant more extensive, prospective studies.
Our data are consistent with previous studies showing frequent CDKN2A/p16 inactivation in EAC, pre-neoplastic BE and dysplastic precursor lesions17, 19, 21, 27–29. CDKN2A/p16 is a member of the INK4 family of cyclin-dependent kinase inhibitor proteins (p16INK4a, p15INK4b, p18INK4c, and p19INK4d) that negatively regulate progression through the G1 phase of the cell cycle by binding to and inhibiting cyclin D/cdk4–6 complexes30. Inactivation of CDKN2A/p16 confers increased susceptibility to a number of human cancers, including pancreatic, esophageal and other carcinomas21, 31, 32 and CDKN2A/p16 plays key roles in senescence and aging33, 34. Our analyses of gene expression by immunohistochemistry for p16 showed that nuclear p16 expression was significantly higher in IM of non-progressors than in IM of progressors, supporting its role in the progression of BE to dysplasia or EAC. Furthermore, p16 nuclear expression was lower in IM of progressors with somatic loss of CDKN2A/B than in IM of progressors without somatic loss of CDKN2A/B, albeit it did not reach statistical significance (P=0.053). In addition to the somatic structural genetic alterations identified in our study, inactivating mutations17, 27, 35, 36 and epigenetic mechanisms such as CpG hypermethylation28, 37 can contribute to reduced p16 expression in some BE cases.
Altered FHIT transcripts have been described in the premalignant stages of EAC development namely in non-dysplastic BE38–40, and FHIT appears to have a pro-apoptotic and tumor growth inhibitory effect in esophageal cancer cells41. We attempted immunohistochemistry for FHIT but the commercial antibodies tested were not satisfactory. Interestingly, in several patients, particularly in the progressor group, the deletions were limited to the first coding exon (exon 5) and might have been missed in previous studies with less sensitive copy number detection methods. Deletion of exon 5 results in loss of FHIT protein, however, the mechanistic implications of FHIT loss in BE progression remain to be well-characterized.
Mutations in TP53 have been detected previously in non-dysplastic pre-progression-BE (46%)27, concurrent-BE (63%17 to 71%21) and dysplasia/EAC (67%21 to 72%42). We identified TP53 mutations by NGS in 31% of our PP-BE samples, but TP53 mutations were present only in patients who also had FHIT or CDKN2A SCNAs, whereas there were no TP53 mutations in the NvDBE1 group (data not shown). In our study we detected TP53 SCNAs in 50% of dysplasia and EAC lesions. However, we did not detect any TP53 SCNAs in pre-progression-BE or in either of the NvDBE cohorts, and found TP53 SCNAs in only 14% of concurrent-BE, suggesting that TP53 SCNAs are alterations that occur later than TP53 point mutations during progression to dysplasia or EAC and may represent a second hit event inactivating TP53 tumor-suppressor function.
We also observed that the number of genomic alterations did not increase from pre-progression to concurrent-BE, suggesting that “molecular dysplasia” in BE at risk of progression can persist for several years, as assessed by genomic alterations in FHIT, CDKN2A and TP53 mutation, whereas TP53 LOH indicates late progression to dysplasia or EAC. These data are consistent with other recent studies19, 21, 22. The SNP-array method used in our study detected genomic alterations in either FHIT, CDKN2A or other somatic deletions in 81% of concurrent-BE cases, suggesting that this assay may be sensitive to predict concurrent dysplasia/EAC that may be missed in routine endoscopic surveillance.
Some previous longitudinal studies of BE progression reported no differences between progressors and non-progressors in FHIT and CDKN2A gene copy number alterations. In the Seattle study22, the study design was focused on late progression from BE to EAC and their non-progressor cohort included several patients with low- and high-grade dysplasia. In a recent study using an NGS panel,27 copy number analysis was limited to chromosomal arm length resolution and the study included several patients initially reported as indefinite for dysplasia or with low-grade dysplasia, which may harbor copy number alterations as demonstrated by us and others17, 22, 38.
In contrast, our study focuses on the early genomic events that occur in histologically confirmed non-dysplastic BE that may underlie progression to dysplasia or cancer, and therefore may implicate mechanistic correlates of the carcinogenic process. The fact that FHIT and CDKN2A copy number alterations are present in significantly higher frequency in non-dysplastic BE of progressors to dysplasia or cancer suggests that these changes may either play a key role in early steps of progression or represent biomarkers of other mechanisms that drive early progression. The presence of FHIT and CDKN2A changes in a small number of non-progressors is not inconsistent with our model and may indicate that: a) the patient is at risk for progression but progression has not yet been observed; b) additional non-detected molecular events may be required for progression or c) progression has occurred but has been eliminated by the host, as dysplasia (especially LGD) may spontaneously resolve in a subset of patients43, 44. The importance of describing early molecular events that may underlie the development of dysplasia is highlighted by current clinical practice guidelines that recommend close follow-up and resection/ablation of dysplastic tissue, including confirmed low-grade dysplasia8.
In summary, our longitudinal study of Barrett’s esophagus progression shows that genomic alterations primarily in FHIT exon 5 and CDKN2A/B are frequently detected in routine FFPE biopsy samples of non-dysplastic Barrett’s epithelium of patients who harbor or develop future dysplasia or EAC, suggesting they may represent important molecular mechanisms underpinning early progression of BE to dysplasia or EAC, and may be further evaluated as biomarkers of progression for routine workup during cancer surveillance in Barrett’s esophagus patients.
Supplementary Material
Novelty and Impact.
This longitudinal study used high-resolution genome-wide SNP-arrays to interrogate large and focal copy number alterations in esophageal samples of patients with Barrett’s intestinal metaplasia. This study uncovered frequent early genomic alterations in non-dysplastic Barrett’s intestinal metaplasia of patients before progression to dysplasia and adenocarcinoma, involving FHIT exon 5 and/or CDKN2A/B, that suggest mechanistic and biomarker roles for these genes in Barrett’s esophagus progression to dysplasia and cancer.
Acknowledgments:
We thank Jacob Shutyak for help with databases and Claudia Cujar for technical help with SNP arrays. The studies were supported in part by grants NCI-R01 CA208711–01 (ARS); BETRNET Center 1 U54CA163004–04 (TCW; ARS); and funds from the Price Foundation (JRS, ARS).
Abbreviations:
- BAF
B-Allele Frequency
- BE
Barrett’s Esophagus
- C-BE
Concurrent Barrett’s Esophagus
- DAC
Dysplasia and Adenocarcinoma of the Esophagus
- EAC
Esophageal Adenocarcinoma
- FFPE
Formalin Fixed, Paraffin Embedded
- H&E
Hematoxylin and Eosin
- HGD
High Grade Dysplasia
- IM
Intestinal Metaplasia
- LGD
Low Grade Dysplasia
- LOH
Loss of Heterozygosity
- MIP
Molecular Inversion Probe
- NvDBE
Never Dysplastic Barrett’s Esophagus
- PP-BE
Pre-progression Barrett’s Esophagus
- SCNAs
Somatic Copy Number Alterations
- SEM
Standard Error of the Mean
- SNP
Single Nucleotide Polymorphism
Footnotes
Conflicts of Interest
No conflicts reported, except for Gary W Falk: Consulting for PAVMED, which is developing a screening device for Barrett’s esophagus that currently has no molecular component
REFERENCES
- 1.Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2010;19: 1468–70. [DOI] [PubMed] [Google Scholar]
- 2.Rustgi AK, El-Serag HB. Esophageal carcinoma. The New England journal of medicine 2014;371: 2499–509. [DOI] [PubMed] [Google Scholar]
- 3.Snider EJ, Freedberg DE, Abrams JA. Potential Role of the Microbiome in Barrett’s Esophagus and Esophageal Adenocarcinoma. Digestive diseases and sciences 2016;61: 2217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pohl J, Pech O, May A, Manner H, Fissler-Eckhoff A, Ell C. Incidence of macroscopically occult neoplasias in Barrett’s esophagus: are random biopsies dispensable in the era of advanced endoscopic imaging? Am J Gastroenterol 2010;105: 2350–6. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68: 7–30. [DOI] [PubMed] [Google Scholar]
- 6.Seer.cancer.gov. Cancer Stat Facts: Esophageal Cancer, 2017.
- 7.Ruol A, Parenti A, Zaninotto G, Merigliano S, Costantini M, Cagol M, Alfieri R, Bonavina L, Peracchia A, Ancona E. Intestinal metaplasia is the probable common precursor of adenocarcinoma in barrett esophagus and adenocarcinoma of the gastric cardia. Cancer 2000;88: 2520–8. [DOI] [PubMed] [Google Scholar]
- 8.Shaheen NJ, Falk GW, Iyer PG, Gerson LB, American College of G. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol 2016;111: 30–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Gastroenterological Association, Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011;140: 1084–91. [DOI] [PubMed] [Google Scholar]
- 10.Sikkema M, de Jonge PJ, Steyerberg EW, Kuipers EJ. Risk of esophageal adenocarcinoma and mortality in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2010;8: 235–44. [DOI] [PubMed] [Google Scholar]
- 11.Hvid-Jensen F, Pedersen L, Drewes AM, Sorensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. The New England journal of medicine 2011;365: 1375–83. [DOI] [PubMed] [Google Scholar]
- 12.Buas MF, He Q, Johnson LG, Onstad L, Levine DM, Thrift AP, Gharahkhani P, Palles C, Lagergren J, Fitzgerald RC, Ye W, Caldas C, et al. Germline variation in inflammation-related pathways and risk of Barrett’s oesophagus and oesophageal adenocarcinoma. Gut 2017;66(10):1739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai TK, Krishnan K, Samala N, Singh J, Cluley J, Perla S, Howden CW. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett’s oesophagus: a meta-analysis. Gut 2012;61: 970–6. [DOI] [PubMed] [Google Scholar]
- 14.Wani S, Falk GW, Post J, Yerian L, Hall M, Wang A, Gupta N, Gaddam S, Singh M, Singh V, Chuang KY, Boolchand V, et al. Risk factors for progression of low-grade dysplasia in patients with Barrett’s esophagus. Gastroenterology 2011;141: 1179–86, 86e1. [DOI] [PubMed] [Google Scholar]
- 15.Wani S, Puli SR, Shaheen NJ, Westhoff B, Slehria S, Bansal A, Rastogi A, Sayana H, Sharma P. Esophageal adenocarcinoma in Barrett’s esophagus after endoscopic ablative therapy: a meta-analysis and systematic review. Am J Gastroenterol 2009;104: 502–13. [DOI] [PubMed] [Google Scholar]
- 16.Caygill CP, Dvorak K, Triadafilopoulos G, Felix VN, Horwhat JD, Hwang JH, Upton MP, Li X, Nandurkar S, Gerson LB, Falk GW. Barrett’s esophagus: surveillance and reversal. Ann N Y Acad Sci 2011;1232: 196–209. [DOI] [PubMed] [Google Scholar]
- 17.Del Portillo A, Lagana SM, Yao Y, Uehara T, Jhala N, Ganguly T, Nagy P, Gutierrez J, Luna A, Abrams J, Liu Y, Brand R, et al. Evaluation of Mutational Testing of Preneoplastic Barrett’s Mucosa by Next-Generation Sequencing of Formalin-Fixed, Paraffin-Embedded Endoscopic Samples for Detection of Concurrent Dysplasia and Adenocarcinoma in Barrett’s Esophagus. The Journal of molecular diagnostics : JMD 2015;17: 412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agrawal N, Jiao Y, Bettegowda C, Hutfless SM, Wang Y, David S, Cheng Y, Twaddell WS, Latt NL, Shin EJ, Wang LD, Wang L, et al. Comparative genomic analysis of esophageal adenocarcinoma and squamous cell carcinoma. Cancer discovery 2012;2: 899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weaver JM, Ross-Innes CS, Shannon N, Lynch AG, Forshew T, Barbera M, Murtaza M, Ong CA, Lao-Sirieix P, Dunning MJ, Smith L, Smith ML, et al. Ordering of mutations in preinvasive disease stages of esophageal carcinogenesis. Nature genetics 2014: 837–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross-Innes CS, Becq J, Warren A, Cheetham RK, Northen H, O’Donovan M, Malhotra S, di Pietro M, Ivakhno S, He M, Weaver JM, Lynch AG, et al. Whole-genome sequencing provides new insights into the clonal architecture of Barrett’s esophagus and esophageal adenocarcinoma. Nature genetics 2015;47: 1038–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stachler MD, Taylor-Weiner A, Peng S, McKenna A, Agoston AT, Odze RD, Davison JM, Nason KS, Loda M, Leshchiner I, Stewart C, Stojanov P, et al. Paired exome analysis of Barrett’s esophagus and adenocarcinoma. Nature genetics 2015;47: 1047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Galipeau PC, Paulson TG, Sanchez CA, Arnaudo J, Liu K, Sather CL, Kostadinov RL, Odze RD, Kuhner MK, Maley CC, Self SG, et al. Temporal and spatial evolution of somatic chromosomal alterations: a case-cohort study of Barrett’s esophagus. Cancer prevention research 2014;7: 114–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster JM, Oumie A, Togneri FS, Vasques FR, Hau D, Taylor M, Tinkler-Hundal E, Southward K, Medlow P, McGreeghan-Crosby K, Halfpenny I, McMullan DJ, et al. Cross-laboratory validation of the OncoScan(R) FFPE Assay, a multiplex tool for whole genome tumour profiling. BMC Med Genomics 2015;8: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gliem TJ, Aypar U. Development of a Chromosomal Microarray Test for the Detection of Abnormalities in Formalin-Fixed, Paraffin-Embedded Products of Conception Specimens. The Journal of molecular diagnostics : JMD 2017;19: 843–7. [DOI] [PubMed] [Google Scholar]
- 25.R Core Team. R: A Language and Environment for Statistical Computing Vienna, Austria: R Foundation for Statistical Computing, 2015. [Google Scholar]
- 26.Makishima H, Maciejewski JP. Pathogenesis and consequences of uniparental disomy in cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2011;17: 3913–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stachler MD, Camarda ND, Deitrick C, Kim A, Agoston AT, Odze RD, Hornick JL, Nag A, Thorner AR, Ducar M, Noffsinger A, Lash RH, et al. Detection of Mutations in Barrett’s Esophagus Before Progression to High-Grade Dysplasia or Adenocarcinoma. Gastroenterology 2018;155: 156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang JS, Guo M, Montgomery EA, Thompson RE, Cosby H, Hicks L, Wang S, Herman JG, Canto MI. DNA promoter hypermethylation of p16 and APC predicts neoplastic progression in Barrett’s esophagus. Am J Gastroenterol 2009;104: 2153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klump B, Hsieh CJ, Holzmann K, Gregor M, Porschen R. Hypermethylation of the CDKN2/p16 promoter during neoplastic progression in Barrett’s esophagus. Gastroenterology 1998;115: 1381–6. [DOI] [PubMed] [Google Scholar]
- 30.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes & development 1999;13: 1501–12. [DOI] [PubMed] [Google Scholar]
- 31.Cowan RW, Maitra A. Genetic progression of pancreatic cancer. Cancer journal 2014;20: 80–4. [DOI] [PubMed] [Google Scholar]
- 32.Wong YF, Chung TK, Cheung TH, Nobori T, Yim SF, Lai KW, Phil M, Yu AL, Diccianni MB, Li TZ, Chang AM. p16INK4 and p15INK4B alterations in primary gynecologic malignancy. Gynecol Oncol 1997;65: 319–24. [DOI] [PubMed] [Google Scholar]
- 33.Martin N, Beach D, Gil J. Ageing as developmental decay: insights from p16(INK4a.). Trends in molecular medicine 2014;20: 667–74. [DOI] [PubMed] [Google Scholar]
- 34.Daniel M, Tollefsbol TO. Epigenetic linkage of aging, cancer and nutrition. The Journal of experimental biology 2015;218: 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardie LJ, Darnton SJ, Wallis YL, Chauhan A, Hainaut P, Wild CP, Casson AG. p16 expression in Barrett’s esophagus and esophageal adenocarcinoma: association with genetic and epigenetic alterations. Cancer letters 2005;217: 221–30. [DOI] [PubMed] [Google Scholar]
- 36.Mokrowiecka A, Wierzchniewska-Lawska A, Smolarz B, Romanowicz-Makowska H, Malecka-Panas E. p16 gene mutations in Barrett’s esophagus in gastric metaplasia - intestinal metaplasia - dysplasia - adenocarcinoma sequence. Advances in medical sciences 2012;57: 71–6. [DOI] [PubMed] [Google Scholar]
- 37.Bian YS, Osterheld MC, Fontolliet C, Bosman FT, Benhattar J. p16 inactivation by methylation of the CDKN2A promoter occurs early during neoplastic progression in Barrett’s esophagus. Gastroenterology 2002;122: 1113–21. [DOI] [PubMed] [Google Scholar]
- 38.Michael D, Beer DG, Wilke CW, Miller DE, Glover TW. Frequent deletions of FHIT and FRA3B in Barrett’s metaplasia and esophageal adenocarcinomas. Oncogene 1997;15: 1653–9. [DOI] [PubMed] [Google Scholar]
- 39.Schildhaus HU, Krockel I, Lippert H, Malfertheiner P, Roessner A, Schneider-Stock R. Promoter hypermethylation of p16INK4a, E-cadherin, O6-MGMT, DAPK and FHIT in adenocarcinomas of the esophagus, esophagogastric junction and proximal stomach. International journal of oncology 2005;26: 1493–500. [PubMed] [Google Scholar]
- 40.Mori M, Mimori K, Shiraishi T, Alder H, Inoue H, Tanaka Y, Sugimachi K, Huebner K, Croce CM. Altered expression of Fhit in carcinoma and precarcinomatous lesions of the esophagus. Cancer research 2000;60: 1177–82. [PubMed] [Google Scholar]
- 41.Ishii H, Dumon KR, Vecchione A, Trapasso F, Mimori K, Alder H, Mori M, Sozzi G, Baffa R, Huebner K, Croce CM. Effect of adenoviral transduction of the fragile histidine triad gene into esophageal cancer cells. Cancer research 2001;61: 1578–84. [PubMed] [Google Scholar]
- 42.Weaver JM, Ross-Innes CS, Shannon N, Lynch AG, Forshew T, Barbera M, Murtaza M, Ong CA, Lao-Sirieix P, Dunning MJ, Smith L, Smith ML, et al. Ordering of mutations in preinvasive disease stages of esophageal carcinogenesis. Nat Genet 2014;46: 837–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phoa KN, van Vilsteren FG, Weusten BL, Bisschops R, Schoon EJ, Ragunath K, Fullarton G, Di Pietro M, Ravi N, Visser M, Offerhaus GJ, Seldenrijk CA, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014;311: 1209–17. [DOI] [PubMed] [Google Scholar]
- 44.Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, Galanko JA, Bronner MP, Goldblum JR, Bennett AE, Jobe BA, Eisen GM, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. The New England journal of medicine 2009;360: 2277–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.