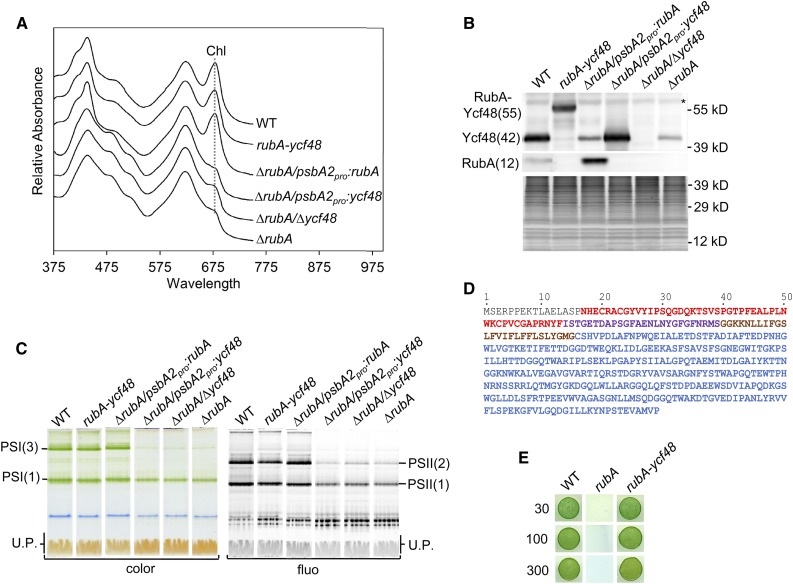
Figure 5.
Comparative Analysis of the Strains Lacking RubA and/or Ycf48 or Expressing a Fused Version of These Proteins.
(A) Whole-cell absorption spectra of the wild type, the strain lacking both RubA and Ycf48 but expressing a RubA-Ycf48 fusion protein (rubA-ycf48), the strain lacking RubA and expressing RubA from the psbA2 promotor (∆rubA/psbA2pro:rubA), the strain lacking RubA and expressing Ycf48 from the psbA2 promotor (∆rubA/psbA2pro:ycf48), the strain lacking both RubA and Ycf48 (∆rubA/∆ycf48), and the strain lacking RubA (∆rubA) were recorded when the photoautotrophic cultures reached an OD of 0.5 at 750 nm; they are shown after shifting for better visibility. The absorption peaks at 679 nm, which reflect Chl content, are designated by a dotted line.
(B) Membranes isolated from strains described in (A) were analyzed by SDS-PAGE. Proteins were subsequently blotted onto a PVDF membrane, and RubA as well as Ycf48 were detected using specific antibodies. For each strain, membranes corresponding to the same number of cells (1.4 × 108) were loaded into the lanes. The SYPRO-stained gel is shown to prove the equal loading of the lanes. The asterisk designates an unspecific cross-reaction. WT, wild type.
(C) Membranes isolated from strains described in (A) were analyzed by CN-PAGE, and the gel was photographed (color) and scanned for Chl fluorescence (fluo). Designation of complexes is as in Figure 2. For each strain, membranes corresponding to the same number of cells (2.5 × 108) were loaded into the lanes. U.P., unassembled proteins. WT, wild type.
(D) Amino acid sequence of the RubA-Ycf48 fusion protein. The RD (red), the rubredoxin linker peptide (purple), the transmembrane helix of RubA (brown), and the Ycf48 part (blue) are shown.
(E) Drops containing 1.25 × 104 cells of the wild type, ∆rubA, or the rubA-ycf48 strain were pipetted on a solid agar plate. The plate was photographed after 3 d of autotrophic growth under constant illumination at 30, 100, or 300 µmol photons m−2 s−1. WT, wild type.