Abstract
Ataxia-telangiectasia is the second most common autosomal recessive hereditary ataxia, with an estimated incidence of 1 in 100,000 births. Besides ataxia and ocular telangiectasias, eye movement abnormalities have long been associated with this disorder and is frequently present in almost all patients. A handful of studies have described the phenomenology of ocular motor deficits in ataxia-telangiectasia. Contemporary literature linked their physiology to cerebellar dysfunction and secondary abnormalities at the level of brainstem. These studies, while providing a proof of concept of ocular motor physiology in disease, i.e. ataxia-telangiectasia, also advanced our understanding of how the cerebellum works. Here, we will summarize the clinical abnormalities seen with ataxia-telangiectasia in each subtype of eye movements and subsequently describe the underlying pathophysiology. Finally we will review how these deficits are linked to abnormal cerebellar function and how it allows better understanding of the cerebellar physiology.
Keywords: Ataxia, eye movement, vestibular, saccade, pursuit, gaze holding
Ataxia-telangiectasia (A-T) is the second most common autosomal recessive hereditary ataxia, with an estimated incidence of 1 in 100,000 births(1). It is a progressive clinical syndrome with multisystem involvement characterized by early onset progressive cerebellar ataxia associated with oculomotor apraxia, choreoathetosis, frequent sino-pulmonary infections, oculocutaneous telangiectasias, and increased risk for hematologic malignancies(2, 4–6). The gene mutation responsible for A-T was mapped to 11q22-23, named ATM in 1995, and sequenced completely by 1996(7,8).
Besides the symptoms that give A-T its name, eye movement abnormalities have long been associated with this disorder, and occur in approximately 88% of patients(4,9–11). With this high prevalence among affected individuals, eye movement dysfunction has served as an important diagnostic clue for A-T(9,12). Numerous reports have attempted to characterize the observed eye movement impairments, with various common conclusions and some discrepancies, largely owing to some reports including only small numbers of cases.
Early report (from 1966) on the ocular manifestations of A-T described three cases from one family in order to summarize the characteristic eye movement dysfunction(13). The key findings were noted as: conjunctival telangiectasias, horizontal conjugate gaze impairment, nystagmus, lack of optokinetic nystagmus, and frequent blinking but with normal vision, pupils and fundus(13). It was found that patients had impairment in initiation of volitional saccades, requiring a head jerk (“thrust”) to force eye deviation and blinking to “reset” the eyes to allow the eyes to finally move into the desired position(13). Aside from this apparent horizontal saccade generation deficit, the patient also displayed impairment in generating quick eye movements to track targets, requiring similar head jerks to accomplish this. Optokinetic nystagmus showed normal amplitude but slow eye movements (13). It was concluded that the eye movement deficits in A-T was more consistent with ataxia rather than what was previously thought of as “ocular motor apraxia”(14,15). The apparent lack of coordination between head movement and eye movement during horizontal saccade generation was thought to be due to cerebellar atrophy. However, the neuropathophysiology of impaired optokinetic nystagmus was difficult to localize at the time due to limited understanding of the pathways mediating the eye movements(13).
In 1981 two cases further correlated eye movement abnormalities in A-T with putative pathophysiology. By this point, it was understood that the ataxia was attributable to cerebellar degeneration, and that the eye movement dysfunction was a form of “supranuclear paralysis”. Here, the characteristic ocular motor impairment was noted to be a progressive loss of voluntary eye movements. Specifically, the patient first lost the ability to generate saccades and had impaired fast phases during vestibular nystagmus. Later, pursuit eye movements became impaired, and eventually “total ophthalmoplegia” occurred (16). Both cases confirmed impaired ability to generate saccades, and additionally, both patients also showed abnormal vertical eye movements. One patient displayed nystagmus upon downgaze. Autopsy of one of the patients confirmed diffuse cerebellar atrophy and loss of Purkinje cells but showed no abnormalities at the ocular motor nuclei, further solidifying the idea that the eye movement impairment in A-T is not due to an inherent loss of eye movements per se, but a more complex “supranuclear” problem (16–18).
With the aid of electro-oculography (EOG), a detailed analysis of eye movements was performed in order to further characterize the types of eye movement dysfunction and to record their prevalence among A-T patients(17, 19). This study took advantage of the phenomenon of “lock ups”, which resulted from an absence of quick phases to reset the eyes during nystagmus and led to the eyes becoming “stuck” at extremes of gaze. The study analyzed the frequency of “lock ups” during optokinetic and vestibular nystagmus among different groups of patients, who were divided based on whether their ocular motor dysfunction was thought to be idiopathic, due to the structural central nervous system abnormalities, neurodegenerative disease (including A-T), perinatal insults, and miscellaneous causes(19). The study found that “lock ups” were virtually universal among patients studied, and they were characterized by intermittent failure in quick phases during optokinetic nystagmus, consistent with a prior report(20). The study also found impairments in smooth pursuit, which were inferred from the presence of catch up saccades on EOG, indicating low pursuit gains(19). By this point, it was known that complex eye movements such as saccades and smooth pursuit were mediated by integrative outputs of several regions in the brain, including frontal eye fields, parietal cortex, basal ganglia, cerebellum, and brainstem (19,21–25).
A study involving a large number of patients with A-T analyzed nine different clinical symptoms and found that ocular motor impairments correlated with increasing age, consistent with the progressive nature of the condition(17). Interestingly, except for pursuit gain and slow phase of optokinetic nystagmus, eye movements analyzed by EOG did not correlate with increasing age. In addition, clinically determined impairment in saccade latency and amplitude worsened with age, but quantitative EOG did not confirm these results(17). Such disparity was attributed to the fact that clinical examination measured volitional saccades while EOG measured reflexive saccades with the patients passively moved by the motorized chair(17).
With improving techniques in studying eye movements and growing understanding of anatomy and physiology of the ocular motor system, the eye movement abnormalities in A-T became a valuable tool in further understanding neuronal pathways regulating eye movements. Contemporary studies utilized A-T as a “disease model” to understand ocular motor physiology. In subsequent section we will first summarize the clinical abnormality noted in each subtype of eye movements. We will describe the contemporary views for the underlying pathophysiology.
Saacades:
Clinical features.
In A-T, both horizontal and vertical saccadic latencies are prolonged in about 77% of patients(17). In addition, both horizontal and vertical saccades were found to be hypometric, so that patients frequently required multiple saccades to complete shifts in gaze, resulting in a step-wise movement of the eyes(17). Electrooculography suggested that peak velocities were higher for the performed (hypometric) saccades amplitude, hoewever, it was appropriately scaled for the intended amplitude of the saccade (i.e. the amplitude of the target shift)(17). It is therefore possible that the hypometric saccades are normally programmed but prematurely interrupted in A-T.
Pathophysiology.
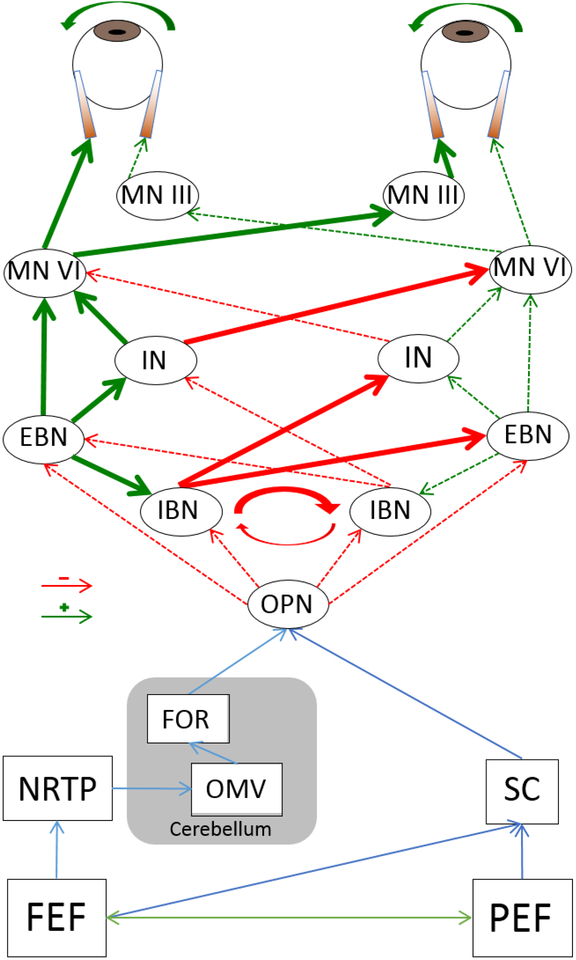
Saccades are under the mediation of at least two collections of neurons (Fig. 1)(25). Premotor burst neurons, which innervate ocular motoneurons (CNIII, VI), are located in different areas of the brain, depending on the direction of eye movements they command. They are further divided into excitatory and inhibitory burst neurons (EBN and IBN, respectively), depending on their output signals. The EBNs generate discharges that allow motoneurons to contract ocular muscle to generate eye movement. They begin discharging at high frequency prior to the initiation of movements, and their firing rate is closely related to the velocity of the saccade, while the total number of spikes corresponds to the saccade amplitude (26–28). The role of IBNs is to suppress activity in antagonistic muscle groups during saccades so that conjugate eye movements are efficiently performed. In addition to supplying antagonistic muscles, the IBNs also inhibit contralateral IBNs, hence creating a reciprocally inhibiting network that is inherently unstable(29,30). Tonically firing omnipause neurons from the nucleus raphe interpositus inhibit EBNs and IBNs keeping the reciprocally inhibiting circuit of oscillation prone burst neurons silent, guaranteeing steady fixation. The omnipause neurons receive fixation related input from the rostral pole of the superior colliculus, the frontal eye fields, as well as the fastigial nucleus, amongst other regions (31–36). While the tonic discharges from the omnipause neurons serve to inhibit saccades in any direction, the transient pauses in their discharge allow burst neurons to initiate saccades. The sustained hyperpolarization of the omnipause neurons is mediated by the inhibitory latch neurons from the PRPF. Impaired latch neuron function results in lack of sustained hyperpolarization of the omnipause neurons, causing eyes to stop prematurely before reaching the target (37–40). It was suspected that dysfunction in the omnipause neurons might cause saccade impairments in A-T. The increased latency, hypometric saccades, and the deliberate blinking that seem to “reset” the eyes that have been reported since early studies all correspond to omnipause neuron impairment (17,25).
Fig 1.
Model of neural network involved in the generation of horizontal saccades. FEF: Frontal Eye Field; PEF: Parietal Eye Field; NRTP: Nucleus Reticularis Tegmenti Pontis; OMV: Oculomotor Vermis; SC: Superior Colliculus; FOR: Fastigial Oculomotor Region; OPN: Omnipause Neuron; IBN: Inhibitory Burst Neuron; EBN: Excitatory Burst Neuron; IN: Interneuron; MN: Motor Neuron.
It is hypothesized that the superior colliculus, the modulator of omnipause neuron and burst neuron, discharges and act as the link between the higher-level commands to generate saccades and the final movement achieved by the eyes. The superior colliculus receives input from higher-level control of saccades, such as the frontal eye fields (41–44). In other words, the impairment in voluntary saccade generation found in A-T point to possible dysfunction in the superior colliculus as well (17,25). Another key source of regulatory input to the omnipause neurons come from the dorsal vermis and fastigial nucleus of the cerebellum (17,25,30). The Purkinje cells of the dorsal vermis, discharge approximately 15ms before saccades are generated in the desired direction (45). Stimulation of the dorsal vermis during already-occurring saccades has been found to modify the trajectory of the saccade, more so than if the stimulation was applied prior to saccade generation. This has led to the hypothesis that the dorsal vermis acts as a feedback modulator of saccades. The fastigial nucleus, which receives input from the dorsal vermis and projects to the omnipause neurons, is another regulator contributing to the integrated production of saccadic movements(33,46,47). The neurons in the fastigial nucleus are thought to contribute to both the initiation and the termination of saccades. Thus, the fastigial nucleus produces commands that accelerate the eyes at the beginning of saccades, but also commands that decelerate the eyes at the termination of saccades(48–50) This is supported by findings that lesions in the superior colliculus or frontal eye fields do not lead to permanent deficits in the precise trajectory and dynamics of saccades, but lesions in the cerebellum, as seen in A-T, can produce saccade dysmetria(46,51–53).
Pursuit:
Clinical features.
In 75% of A-T subjects the smooth pursuit is found to be abnormally slow and marked by frequent “catch-up” saccades(17). In addition, pursuit gain is lower than normal (17).
Pathophysiology.
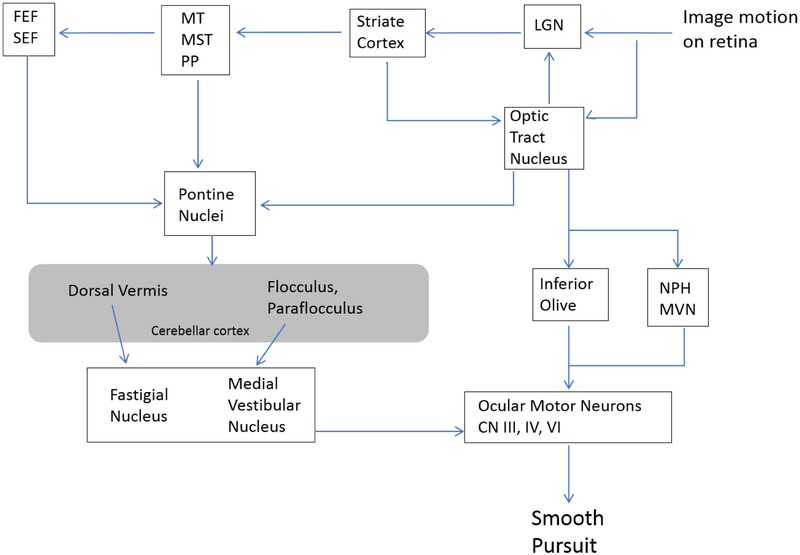
While several areas of the brain such as the primary visual cortex, middle temporal area, frontal and supplementary eye fields might contribute to smooth pursuit, the pursuit pathway is thought to converge, via the pontine nuclei, before projecting to the cerebellum, which in turn projects to the premotor areas in the brain stem (Fig. 2) (54–61). Although the flocculus and parafloccus both receive projections from the pontine nuclei, the ventral paraflocculus is more important for smooth pursuit(62,63). It is hypothesized that other regions of the cerebellum, such as the dorsal vermis and the caudal fastigial nucleus, also contribute to smooth pursuit (47,63,64). The dorsal vermis has been shown to encode target velocity in space. The lesions of the dorsal vermis impair the onset of smooth pursuit. The fastigial nucleus ocular motor region (FOR) also contributes to the acceleration phase of smooth pursuit onset. Based on their pattern of discharge, at the onset of smooth pursuit, it is hypothesized that these neurons help accelerate the eyes during initiation of pursuit, similar to their activity during saccades (65–69). Unilateral lesions show decreased impairment in pursuit onset and sustained pursuit in all directions, but bilateral lesions do not affect pursuit onset but still impair sustained pursuit (70,71). Overall, the dorsal vermis and FOR play a more important role in onset of pursuit, while the flocculus and paraflocculus mediates tracking after pursuit has been initiated (25). A-T is known to affect multiple cerebellar lobules, leading to Purkinje neuron degeneration. Therefore, like many other cerebellar disorders, A-T leads to pursuit abnormalities.
Fig 2.
Proposed schema of the neural network for smooth-pursuit eye movements, allowing pursuit of moving images on the retina. FEF: Frontal Eye Field; SEF: Supplementary Eye Field; MT: Middle Temporal visual area; MST: Medial Superior Temporal visual area; LGN: Lateral Geniculate Nucleus; NPH: Nucleus Prepositus; MVN: Medial Vestibular Nucleus.
Gaze holding deficits:
Clinical features.
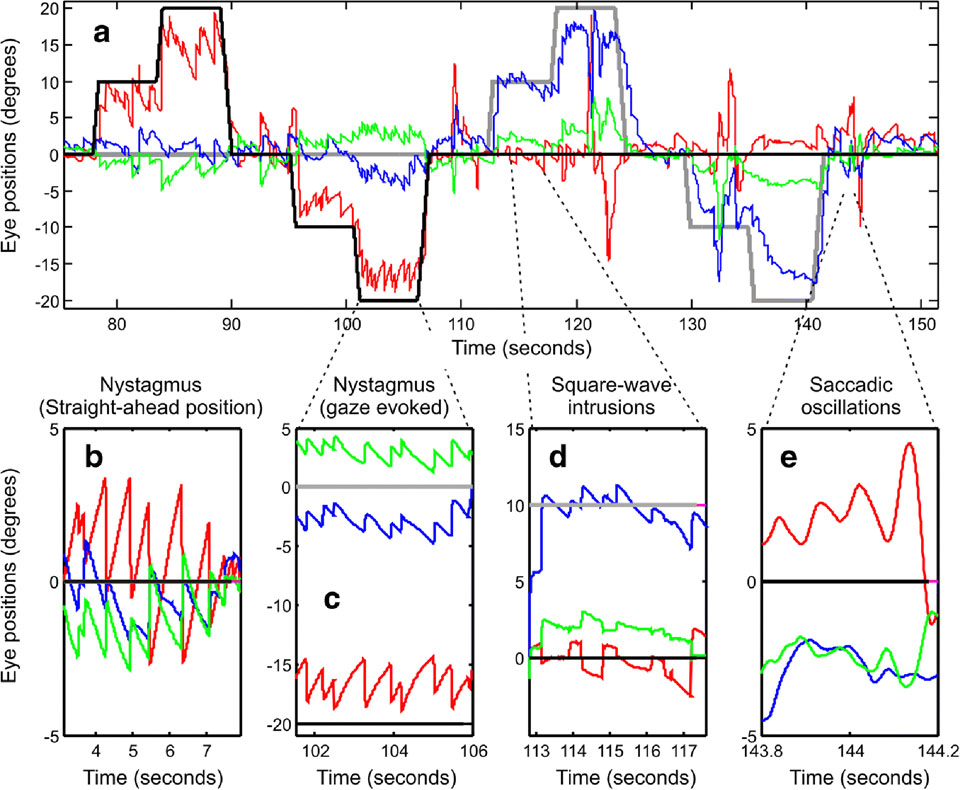
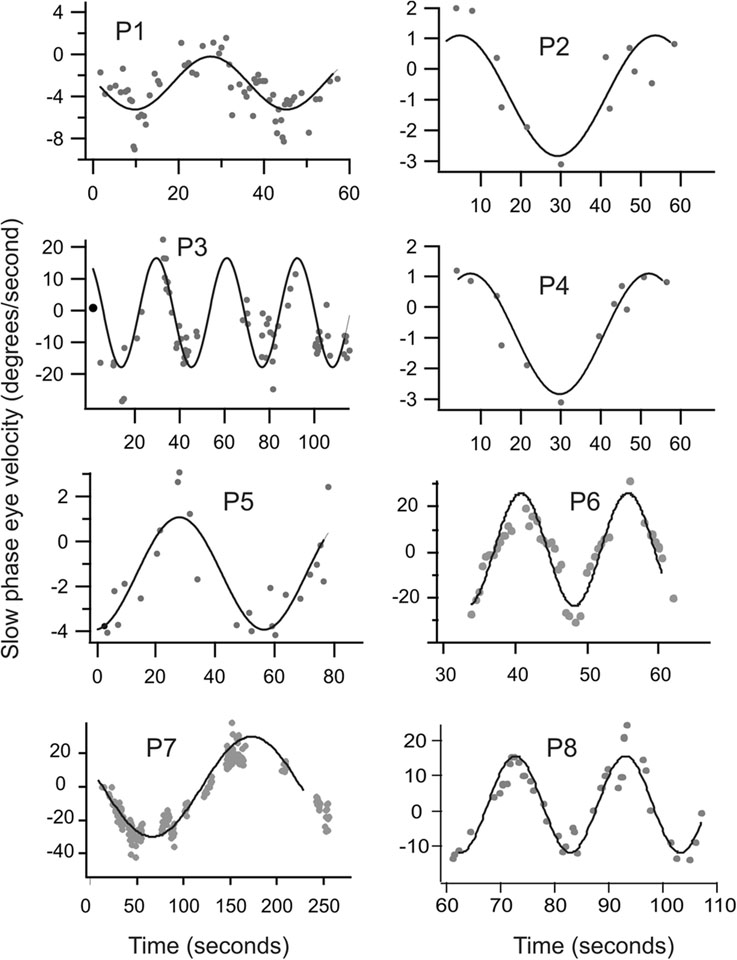
During gaze fixation, A-T patients are found to have abnormal eye movements, including nystagmus and saccadic intrusions (30)(Fig. 3). Spontaneous horizontal and downbeat nystagmus (when eyes are in central position, Fig 3B) and horizontal, vertical, or their combination leading to oblique gaze-evoke nystagmus (when the eyes are directed away from center, Fig 3C) were found(30). During attempted straight-ahead fixation, some patients had spontaneous periodic alternating nystagmus (PAN), where the direction of nystagmus alternated, while others were found to have non-periodic spontaneous nystagmus(30). PAN refers to spontaneous horizontal nystagmus that reverses directions with a set period of time, approximately around 2 minutes. A-T patients were found to have cycles of PAN lasting between 15 seconds to 4 minutes, displaying a wide inter-patient range but relatively stable person-specific periods(30)(Fig 4). The saccadic intrusions included micro-saccadic oscillations and square-wave saccadic intrusions(30)(Fig 3C,D).
Fig 3.
An example of a gaze fixation in one ataxia-telangiectasia patient. (A) Horizontal, vertical, and torsional eye positions (y-axis) are plotted versus time (x-axis). Horizontal eye positions are plotted in red color, blue trace is vertical eye position, and torsional is green. Black dashed line represents the horizontal target position and the grey dashed line is the vertical target position. Positive values correspond right-ward, up-ward, and clock-wise rotations from the subjects’ viewpoint. The detailed qualitative inspection of the eye movement waveform is depicted in panels ‘B-E’. Arrows in panels ‘D’ and ‘E’ illustrate square-wave saccadic intrusions and saccadic oscillations, respectively (reproduced with permission from Shaikh, et al., 2009).
Fig 4.
Quantitative characteristics of periodic alternating nystagmus (PAN) that was manifest in eight ataxia-telangiectasia patients. Each panel represents one patient and each data point represents the slow phase eye velocity of the drift phase of the nystagmus. Data points with the negative sign represent the velocity of the left-ward drifts. The black line in each panel represents the best sinusoidal fit to the slow phase eye velocity. Negative values of eye velocity represent leftward movement (reproduced with permission from Shaikh et al., 2009).
Pathophysiology.
The lesion responsible for PAN is thought to be the vestibular velocity storage mechanism, residing in the nodulus and uvula of the cerebellum. This mechanism stores movement velocity in sustained head rotation in order to overcome habituation in the semicircular canals, so that vestibular nystagmus can continue as needed(25,72). With lesions in these areas, the duration of nystagmus is prolonged, inducing a vestibular adaptive system that reverses the direction of nystagmus(30). In cases of cerebellar lesions, the instability in the velocity storage system and subsequent adaptive mechanism is thought to generate the alternating direction of the nystagmus(25,30,47,73,74). The non-periodic spontaneous horizontal nystagmus is thought to result from the vestibular bias coexisting with gaze holding impairments, which shift the null position of central gaze(30). The downbeat nystagmus may have a multifactorial pathophysiology, possibly due to lesions in the pons or caudal medulla(30).
The saccadic oscillations observed in A-T patients are thought to result from instability in the membranes of saccadic burst neurons(30). Loss of Purkinje cells can disinhibit the FOR, which in turn projects to the omnipause neurons, as discussed previously. If the omnipause neurons lose their ability to tonically suppress the EBNs and IBNs, increased burst generation in these neurons could lead to spontaneous saccadic movements(30). Square wave saccadic intrusions are a common finding in healthy subjects, but in A-T patients, their amplitudes are abnormally large(25,30). Square-wave jerks, in addition to A-T, are seen in variety of neurodegenerative conditions including cortical degenerations leading to dementia, some forms of genetically dominant spinocerebellar ataxias (25). Physiology of squarewave jerks is unclear in non-cerebellar disorders, one possibility is that they are the results of abnormally large fixational eye movement called microsaccade(25). Role of cerebellum in pathogenesis of square-wave intrusions was also implicated, particularly in context of A-T. It was hypothesized that the loss of inhibition of FOR by Purkinje cells in the cerebellum allows periodic firing of the FOR due to intrinsic pacemaker properties of the neurons – i.e. they spontaneously discharge if not inhibited(30). Since FOR discharge stimulate contraversive but inhibit ipsiversive saccades, if one FOR discharged without matching activity from the contralateral side, saccadic intrusions can occur(30). This could also help explain the amplitude of saccadic intrusions, which correlates with the degree of disinhibition(30).
Vestibulo-ocular reflex
Clinical features.
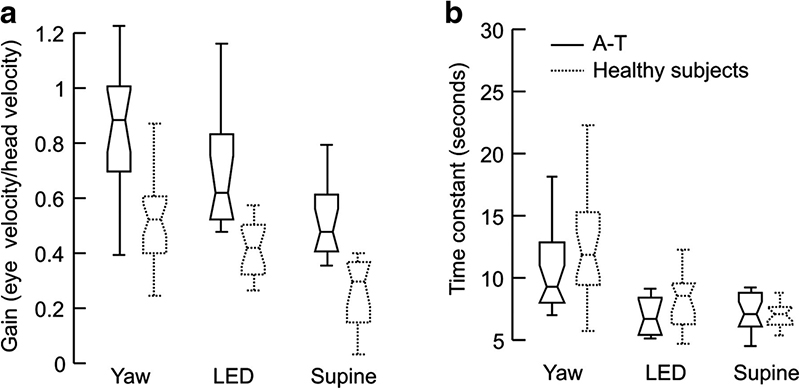
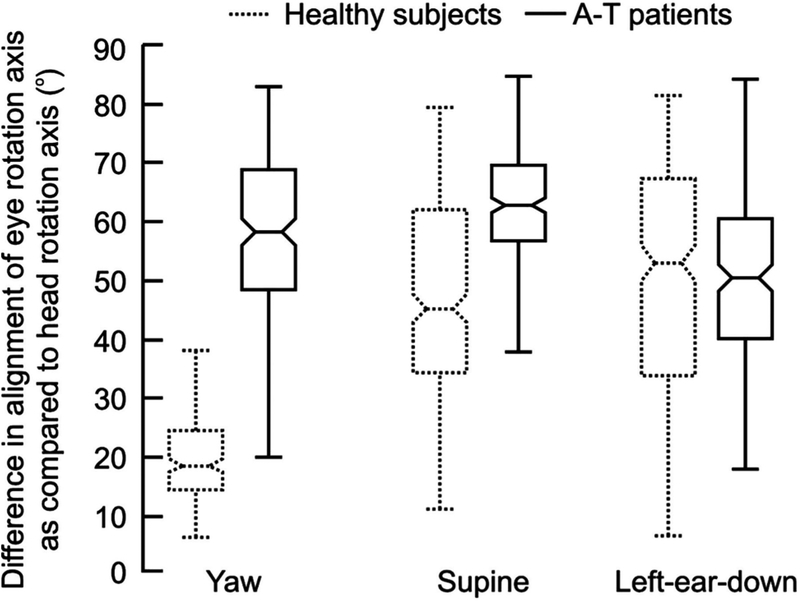
Several studies suggested abnormal vestibular function in A-T (18,75,76). In one relatively recent study, 13 patients with A-T had abnormalities in several key vestibular functions (76). The VOR gain was found to be increased in all subjects. VOR time constant is expected to be prolonged when cerebellar nodulus and ventral uvula are damaged(77)(Fig. 5A). On the contrary, in ataxia telangiectasia, the VOR time constant was shortened compared to normal(76)(Fig. 5B). In addition, the axis of eye rotation was not aligned with the axis of head rotation (Fig. 6). As a result, prominent VOR cross-coupling, abnormal eye movements directed along axes orthogonal to that of head rotation, were observed. The cross-coupled VOR in addition to increased gain of the VOR could manifest in symptomatic vertigo and imbalance in these patients.
Fig 5.
Panel A compares the VOR gain of ataxia-telangiectasis (A-T) patients (solid box and whisker plots) to healthy control subjects (dotted box and whisker plots) during all three planes of rotation. B: comparison of the VOR time constant between the two groups (reproduced with permission from Shaikh, et al., 2011).
Fig 6.
Summary of differences in the angular orientation of the eye movement and head movement axes. The difference in rotation axes is plotted on y-axis. Each box and whisker plot shows one condition in a given group of 13 ataxia-telangiectasia patients (solid box) and 11 normal subjects (dashed box). A-T: ataxia telangiectasia (reproduced with permission from Shaikh, et al., 2011).
Pathophysiology.
The vestibular reflexes guarantee clear vision during locomotion. Evidence of this principle comes from the individuals who have abnormal vestibular reflexes: these patients experience diminished visual acuity with head motion and postural instability. Experimental evidence has supported the role of vestibulocerebellum in long-term adaptive control of VOR that guarantees precise matching of the eye position to the timing, inverse amplitude, and direction of the head motion(78–82). For example, during motion, the head orientation dynamically changes with respect to gravity. In order to stabilize the gaze and thus facilitate clear vision, the axis of eye rotation of the VOR must remain parallel to the axis of head rotation. Macaque studies have shown that unlike vestibular afferents(83) and some vestibular and deep cerebellar cells(84–86), cerebellar Purkinje and granule cells from the nodulus and ventral uvula encode vestibular signals in a space-fixed frame of reference (87). Therefore, it is expected that dynamic reorientation of VOR axis in response to head motion is impaired if cerebellar nodulus is experimentally lesioned(88,89). Impaired VOR gain in A-T can be described by combination of two mechanisms. One is impaired inhibitory input from the GABAergic Purkinje projections causing disinhibition of the vestibular nuclei, while the second is impaired cerebellar-dependent adaptation(78,82,90). Abnormal directional tuning of the VOR (VOR cross-coupling) could be described by the lesion of the vestibulocerebellum(79–81). VOR cross-coupling can be described by several pathophysiological mechanisms. One involves the lesions of the cerebellar nodulus and uvula, the regions that are thought to play a critical role in determining the internal estimate of gravitational force and assuring the alignment of eye movement axis with the head movement (88,89,91) The second mechanism emphasizes the loss of cerebellar-mediated adjustment to the relative synaptic strength of the converging canal inputs in the vestibular nucleus(92) that normally compensates for anatomic misalignments between the semicircular canals and the pulling directions of the orbital muscles (80,92). It was speculated that that optimally calibrated output of the vestibular velocity storage during the given plane of rotation plays an important role in attenuating unwanted cross-coupled VOR responses.
In order to faithfully compensate for the rapid decay of vestibular coding of prolonged low-frequency rotation a mechanism called velocity storage increases the VOR bandwidth(93). Cerebellar nodulus and ventral uvula plays a critical role in controlling the velocity storage, lesions of these cerebellar areas increase the VOR time constant measured during pre- and post-rotational nystagmus(77). Given prominent involvement of the cerebellar nodulus in A-T, we expect prominent VOR impairments. Unlike experimental lesions of nodulus and ventral uvula in monkeys, in human patients with degenerative cerebellar lesions the VOR time constant may or may not be increased. The length of time constant depends on the extent of the lesion and co-existing brainstem involvement in A-T(76).
The direct implication of the study of rare disorders of eye movements, such as A-T, is may be important in understanding how brain and the cerebellum in particular controls movements. Although the eye movement impairments in A-T serve as a valuable tool in testing mechanistic underpinning of the given disorder(25,76,94); A-T is a degenerative disorder, it may not always represent findings that are consistent with focal lesion studies in animal models.
Acknowledgements:
This work was supported by Dystonia Coalition Career Development Award (AS), Dystonia Medical Research Foundation Research Grant (AS), and the American Academy of Neurology Career Development Award (AS). There are no conflict of interests.
Footnotes
Conflict of interest:
The authors have no conflict of interest.
References:
- 1.Swift M, Morrell D, Cromartie E, Chamberlin AR, Skolnick MH, Bishop DT. The incidence and gene frequency of ataxia-telangiectasiaA-T in the United States. Am J Hum Genet. 1986. November;39(5):573–83. [PMC free article] [PubMed] [Google Scholar]
- 2.Jayadev S, Bird TD. Hereditary ataxias: overview. Genet Med. 2013. September;15(9):673–83. [DOI] [PubMed] [Google Scholar]
- 3.Teller WM, Millichap JG. Ataxia-TelangiectasiaA-T (Louis-Bar Syndrome) with Prominent Sinopulmonary Disease. JAMA. 1961. March 4;175(9):779–82. [DOI] [PubMed] [Google Scholar]
- 4.Boder E, Sedgwick RP. ATAXIA-TELANGIECTASIAA-T: A Familial Syndrome of Progressive Cerebellar Ataxia, Oculocutaneous Telangiectasia and Frequent Pulmonary Infection. Pediatrics. 1958. April 1;21(4):526–54. [PubMed] [Google Scholar]
- 5.McFARLIN DE, Strober W, Waldmann TA. ATAXIA-TELANGIECTASIAA-T. Medicine (Baltimore). 1972. July;51(4):281. [DOI] [PubMed] [Google Scholar]
- 6.Biemond A Paleo Cerebellar atrophy with extrapyramidal manifestations in association with bronchiectasis and telangiectasia of the conjunctiva bulbi as a familial syndrome. Van Bogaert Radermecker J Eds Proc First Int Congr. 1957;(206). [Google Scholar]
- 7.Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995. June 23;268(5218):1749–53. [DOI] [PubMed] [Google Scholar]
- 8.Byrd PJ, McConville CM, Cooper P, Parkhill J, Stankovic T, McGuire GM, et al. Mutations revealed by sequencing the 5’ half of the gene for ataxia telangiectasia. Hum Mol Genet. 1996. January;5(1):145–9. [DOI] [PubMed] [Google Scholar]
- 9.Moin M, Aghamohammadi A, Kouhi A, Tavassoli S, Rezaei N, Ghaffari S-R, et al. Ataxia telangiectasiaA-T in Iran: clinical and laboratory features of 104 patients. Pediatr Neurol. 2007. July;37(1):21–8. [DOI] [PubMed] [Google Scholar]
- 10.Smith JL, Cogan DG. Ataxia-TelangiectasiaA-T. AMA Arch Ophthalmol. 1959. September 1;62(3):364–9. [Google Scholar]
- 11.Cogan DG, Chu FC, Reingold D, Barranger J. Ocular Motor Signs in Some Metabolic Diseases. Arch Ophthalmol. 1981. October 1;99(10):1802–8. [DOI] [PubMed] [Google Scholar]
- 12.Federighi P, Ramat S, Rosini F, Pretegiani E, Federico A, Rufa A. Characteristic Eye Movements in Ataxia-Telangiectasia-Like Disorder: An Explanatory Hypothesis. Front Neurol. 2017. November 9;8:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyams SW, Reisner SH, Neumann E. The Eye Signs In Ataxia-TelangiectasiaA-T. Am J Ophthalmol. 62(6):1118–24. [DOI] [PubMed] [Google Scholar]
- 14.Smith JL, Cogan DG. Ataxia-TelangiectasiaA-T. AMA Arch Ophthalmol. 1959. September 1;62(3):364–9. [Google Scholar]
- 15.Boder E, Sedgwick RP. Ataxiatelangiectasia: A review of 150 cases. Intern Cong Ment Retard. 1964; [Google Scholar]
- 16.Cogan DG, Chu FC, Reingold D, Barranger J. Ocular Motor Signs in Some Metabolic Diseases. Arch Ophthalmol. 1981. October 1;99(10):1802–8. [DOI] [PubMed] [Google Scholar]
- 17.Lewis Richard F., Lederman Howard M., Crawford Thomas O. Ocular motor abnormalities in ataxia telangiectasia. Ann Neurol. 1999;46(3):287–95. [DOI] [PubMed] [Google Scholar]
- 18.Baloh RW, Yee RD, Boder E. Eye movements in ataxia-telangiectasia. Neurology. 1978. November 1;28(11):1099–1099. [DOI] [PubMed] [Google Scholar]
- 19.Harris CM, Shawkat F, Russell-Eggitt I, Wilson J, Taylor D. Intermittent horizontal saccade failure (‘ocular motor apraxia’) in children. Br J Ophthalmol. 1996. February;80(2):151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zee DS, Yee RD, Singer HS. Congenital ocular motor apraxia. Brain J Neurol. 1977. September;100(3):581–99. [DOI] [PubMed] [Google Scholar]
- 21.Reed H, Israels S. Congenital Ocular Motor Apraxia: A Form of Horizontal Gaze Palsy. Br J Ophthalmol. 1956. July 1;40(7):444–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riopel DA. Congenital Ocular Motor Apraxia* *From the Division of Ophthalmology, College of Medicine, University of Florida. Am J Ophthalmol. 1963. March;55(3):511–4. [PubMed] [Google Scholar]
- 23.ROBLES J Congenital ocular motor apraxia in identical twins. Arch Ophthalmol. 1966. June 1;75(6):746–9. [DOI] [PubMed] [Google Scholar]
- 24.Orrison WW, Robertson WC. Congenital Ocular Motor Apraxia: A Possible Disconnection Syndrome. Arch Neurol. 1979. January 1;36(1):29–31. [DOI] [PubMed] [Google Scholar]
- 25.Leigh RJ, Zee DS. The Neurology of Eye Movements. Oxford University Press; 2015. 1137 p. [Google Scholar]
- 26.Van Gisbergen JA, Robinson DA, Gielen S. A quantitative analysis of generation of saccadic eye movements by burst neurons. J Neurophysiol. 1981. March;45(3):417–42. [DOI] [PubMed] [Google Scholar]
- 27.Hepp K, Henn V. Spatio-temporal recoding of rapid eye movement signals in the monkey paramedian pontine reticular formation (PPRF). Exp Brain Res. 1983;52(1):105–20. [DOI] [PubMed] [Google Scholar]
- 28.Henn V, Hepp K, Vilis T. Rapid eye movement generation in the primate. Physiology, pathophysiology, and clinical implications. Rev Neurol (Paris). 1989;145(8–9):540–5. [PubMed] [Google Scholar]
- 29.Ramat S, Leigh RJ, Zee DS, Optican LM. Ocular oscillations generated by coupling of brainstem excitatory and inhibitory saccadic burst neurons. Exp Brain Res. 2005. January;160(1):89–106. [DOI] [PubMed] [Google Scholar]
- 30.Shaikh AG, Marti S, Tarnutzer AA, Palla A, Crawford TO, Straumann D, et al. Gaze fixation deficits and their implication in ataxia-telangiectasiaA-T. J Neurol Neurosurg Psychiatry. 2009. August;80(8):858–64. [DOI] [PubMed] [Google Scholar]
- 31.Shook BL, Schlag-Rey M, Schlag J. Direct projection from the supplementary eye field to the nucleus raphe interpositus. Exp Brain Res. 1988. October 1;73(1):215–8. [DOI] [PubMed] [Google Scholar]
- 32.Stanton GB, Goldberg ME, Bruce CJ. Frontal eye field efferents in the macaque monkey: I. Subcortical pathways and topography of striatal and thalamic terminal fields. J Comp Neurol. 1988. May 22;271(4):473–92. [DOI] [PubMed] [Google Scholar]
- 33.Noda H, Sugita S, Ikeda Y. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J Comp Neurol. 1990. December 8;302(2):330–48. [DOI] [PubMed] [Google Scholar]
- 34.Büttner-Ennever JA, Horn AK, Henn V, Cohen B. Projections from the superior colliculus motor map to omnipause neurons in monkey. J Comp Neurol. 1999. October 11;413(1):55–67. [DOI] [PubMed] [Google Scholar]
- 35.Gandhi NJ, Keller EL. Spatial distribution and discharge characteristics of superior colliculus neurons antidromically activated from the omnipause region in monkey. J Neurophysiol. 1997. October;78(4):2221–5. [DOI] [PubMed] [Google Scholar]
- 36.Gandhi NJ, Keller EL. Activity of the brain stem omnipause neurons during saccades perturbed by stimulation of the primate superior colliculus. J Neurophysiol. 1999. December;82(6):3254–67. [DOI] [PubMed] [Google Scholar]
- 37.Ohgaki T, Markham CH, Schneider JS, Curthoys IS. Anatomical evidence of the projection of pontine omnipause neurons to midbrain regions controlling vertical eye movements. J Comp Neurol. 1989. November 22;289(4):610–25. [DOI] [PubMed] [Google Scholar]
- 38.Keller EL, Edelman JA. Use of interrupted saccade paradigm to study spatial and temporal dynamics of saccadic burst cells in superior colliculus in monkey. J Neurophysiol. 1994. December;72(6):2754–70. [DOI] [PubMed] [Google Scholar]
- 39.Keller EL, Gandhi NJ, Shieh JM. Endpoint accuracy in saccades interrupted by stimulation in the omnipause region in monkey. Vis Neurosci. 1996. December;13(6):1059–67. [DOI] [PubMed] [Google Scholar]
- 40.Munoz DP, Waitzman DM, Wurtz RH. Activity of neurons in monkey superior colliculus during interrupted saccades. J Neurophysiol. 1996. June;75(6):2562–80. [DOI] [PubMed] [Google Scholar]
- 41.Schiller PH, True SD, Conway JL. Deficits in eye movements following frontal eye-field and superior colliculus ablations. J Neurophysiol. 1980. December;44(6):1175–89. [DOI] [PubMed] [Google Scholar]
- 42.Schiller PH, Chou IH. The effects of frontal eye field and dorsomedial frontal cortex lesions on visually guided eye movements. Nat Neurosci. 1998. July;1(3):248–53. [DOI] [PubMed] [Google Scholar]
- 43.Dias EC, Segraves MA. Muscimol-induced inactivation of monkey frontal eye field: effects on visually and memory-guided saccades. J Neurophysiol. 1999. May;81(5):2191–214. [DOI] [PubMed] [Google Scholar]
- 44.Hanes DP, Smith MK, Optican LM, Wurtz RH. Recovery of saccadic dysmetria following localized lesions in monkey superior colliculus. Exp Brain Res. 2005. January;160(3):312–25. [DOI] [PubMed] [Google Scholar]
- 45.Ohtsuka K, Noda H. Discharge properties of Purkinje cells in the oculomotor vermis during visually guided saccades in the macaque monkey. J Neurophysiol. 1995. November;74(5):1828–40. [DOI] [PubMed] [Google Scholar]
- 46.Optican LM, Robinson DA. Cerebellar-dependent adaptive control of primate saccadic system. J Neurophysiol. 1980. December;44(6):1058–76. [DOI] [PubMed] [Google Scholar]
- 47.Zee DS, Yamazaki A, Butler PH, Gücer G. Effects of ablation of flocculus and paraflocculus of eye movements in primate. J Neurophysiol. 1981. October;46(4):878–99. [DOI] [PubMed] [Google Scholar]
- 48.Ohtsuka K, Noda H. Saccadic burst neurons in the oculomotor region of the fastigial nucleus of macaque monkeys. J Neurophysiol. 1991. June;65(6):1422–34. [DOI] [PubMed] [Google Scholar]
- 49.Fuchs AF, Robinson FR, Straube A. Role of the caudal fastigial nucleus in saccade generation. I. Neuronal discharge pattern. J Neurophysiol. 1993. November;70(5):1723–40. [DOI] [PubMed] [Google Scholar]
- 50.Helmchen C, Straube A, Büttner U. Saccade-related activity in the fastigial oculomotor region of the macaque monkey during spontaneous eye movements in light and darkness. Exp Brain Res. 1994;98(3):474–82. [DOI] [PubMed] [Google Scholar]
- 51.Selhorst JB, Stark L, Ochs AL, Hoyt WF. Disorders in cerebellar ocular motor control. I. Saccadic overshoot dysmetria. An oculographic, control system and clinico-anatomical analysis. Brain J Neurol. 1976. September;99(3):497–508. [DOI] [PubMed] [Google Scholar]
- 52.Selhorst JB, Stark L, Ochs AL, Hoyt WF. Disorders in cerebellar ocular motor control. II. Macrosaccadic oscillation. An oculographic, control system and clinico-anatomical analysis. Brain J Neurol. 1976. September;99(3):509–22. [DOI] [PubMed] [Google Scholar]
- 53.Robinson FR, Straube A, Fuchs AF. Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. J Neurophysiol. 1993. November;70(5):1741–58. [DOI] [PubMed] [Google Scholar]
- 54.Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988. May 6;240(4853):740–9. [DOI] [PubMed] [Google Scholar]
- 55.Glickstein M, Gerrits N, Kralj-Hans I, Mercier B, Stein J, Voogd J. Visual pontocerebellar projections in the macaque. J Comp Neurol. 1994. November 1;349(1):51–72. [DOI] [PubMed] [Google Scholar]
- 56.Van Essen DC, Gallant JL. Neural mechanisms of form and motion processing in the primate visual system. Neuron. 1994. July 1;13(1):1–10. [DOI] [PubMed] [Google Scholar]
- 57.Ilg UJ. Commentary: smooth pursuit eye movements: from low-level to high-level vision. Prog Brain Res. 2002;140:279–98. [DOI] [PubMed] [Google Scholar]
- 58.Werner JS, Chalupa LM, editors. The New Visual Neurosciences. 1 edition Cambridge, Massachusetts: The MIT Press; 2013. 1696 p. [Google Scholar]
- 59.Derrington AM, Allen HA, Delicato LS. Visual Mechanisms of Motion Analysis and Motion Perception. Annu Rev Psychol. 2004;55(1):181–205. [DOI] [PubMed] [Google Scholar]
- 60.Vaina LM, Soloviev S. First-order and second-order motion: neurological evidence for neuroanatomically distinct systems. Prog Brain Res. 2004;144:197–212. [DOI] [PubMed] [Google Scholar]
- 61.Chen KJ, Sheliga BM, Fitzgibbon EJ, Miles FA. Initial ocular following in humans depends critically on the fourier components of the motion stimulus. Ann N Y Acad Sci. 2005. April;1039:260–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagao S, Kitamura T, Nakamura N, Hiramatsu T, Yamada J. Differences of the primate flocculus and ventral paraflocculus in the mossy and climbing fiber input organization. J Comp Neurol. 1997. June 16;382(4):480–98. [DOI] [PubMed] [Google Scholar]
- 63.Rambold H, Churchland A, Selig Y, Jasmin L, Lisberger SG. Partial Ablations of the Flocculus and Ventral Paraflocculus in Monkeys Cause Linked Deficits in Smooth Pursuit Eye Movements and Adaptive Modification of the VOR. J Neurophysiol. 2002. February;87(2):912–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heinen SJ, Keller EL. The function of the cerebellar uvula in monkey during optokinetic and pursuit eye movements: single-unit responses and lesion effects. Exp Brain Res. 1996. June;110(1):1–14. [DOI] [PubMed] [Google Scholar]
- 65.Kase M, Noda H, Suzuki DA, Miller DC. Target velocity signals of visual tracking in vermal Purkinje cells of the monkey. Science. 1979. August 17;205(4407):717–20. [DOI] [PubMed] [Google Scholar]
- 66.Suzuki DA, Noda H, Kase M. Visual and pursuit eye movement-related activity in posterior vermis of monkey cerebellum. J Neurophysiol. 1981. November;46(5):1120–39. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki DA, Keller EL. The role of the posterior vermis of monkey cerebellum in smooth-pursuit eye movement control. II. Target velocity-related Purkinje cell activity. J Neurophysiol. 1988. January;59(1):19–40. [DOI] [PubMed] [Google Scholar]
- 68.Ohtsuka K, Enoki T. Transcranial magnetic stimulation over the posterior cerebellum during smooth pursuit eye movements in man. Brain J Neurol. 1998. March;121 ( Pt 3):429–35. [DOI] [PubMed] [Google Scholar]
- 69.Shinmei Y, Yamanobe T, Fukushima J, Fukushima K. Purkinje cells of the cerebellar dorsal vermis: simple-spike activity during pursuit and passive whole-body rotation. J Neurophysiol. 2002. April;87(4):1836–49. [DOI] [PubMed] [Google Scholar]
- 70.Fuchs AF, Robinson FR, Straube A. Participation of the caudal fastigial nucleus in smooth-pursuit eye movements. I. Neuronal activity. J Neurophysiol. 1994. December;72(6):2714–28. [DOI] [PubMed] [Google Scholar]
- 71.Vahedi K, Rivaud S, Amarenco P, Pierrot-Deseilligny C. Horizontal eye movement disorders after posterior vermis infarctions. J Neurol Neurosurg Psychiatry. 1995. January 1;58(1):91–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laurens J, Angelaki DE. The functional significance of velocity storage and its dependence on gravity. Exp Brain Res. 2011. May;210(3–4):407–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zee DS, Leigh RJ, Mathieu-Millaire F. Cerebellar control of ocular gaze stability. Ann Neurol. 1980. January;7(1):37–40. [DOI] [PubMed] [Google Scholar]
- 74.Leigh RJ, Robinson DA, Zee DS. A hypothetical explanation for periodic alternating nystagmus: instability in the optokinetic-vestibular system. Ann N Y Acad Sci. 1981;374:619–35. [DOI] [PubMed] [Google Scholar]
- 75.Stell R, Bronstein AM, Plant GT, Harding AE. Ataxia telangiectasia: a reappraisal of the ocular motor features and their value in the diagnosis of atypical cases. Mov Disord Off J Mov Disord Soc. 1989;4(4):320–9. [DOI] [PubMed] [Google Scholar]
- 76.Shaikh AG, Marti S, Tarnutzer AA, Palla A, Crawford TO, Straumann D, et al. Ataxia telangiectasia: a “disease model” to understand the cerebellar control of vestibular reflexes. J Neurophysiol. 2011. June;105(6):3034–41. [DOI] [PubMed] [Google Scholar]
- 77.Waespe W, Cohen B, Raphan T. Dynamic modification of the vestibulo-ocular reflex by the nodulus and uvula. Science. 1985. April 12;228(4696):199–202. [DOI] [PubMed] [Google Scholar]
- 78.Ito M Neurophysiological aspects of the cerebellar motor control system. Int J Neurol. 1970;7(2):162–76. [PubMed] [Google Scholar]
- 79.Schultheis LW, Robinson DA. Directional plasticity of the vestibuloocular reflex in the cat. Ann N Y Acad Sci. 1981;374:504–12. [DOI] [PubMed] [Google Scholar]
- 80.Walker MF, Zee DS. Cerebellar disease alters the axis of the high-acceleration vestibuloocular reflex. J Neurophysiol. 2005. November;94(5):3417–29. [DOI] [PubMed] [Google Scholar]
- 81.Walker MF, Zee DS. Directional abnormalities of vestibular and optokinetic responses in cerebellar disease. Ann N Y Acad Sci. 1999. May 28;871:205–20. [DOI] [PubMed] [Google Scholar]
- 82.Lisberger SG. The latency of pathways containing the site of motor learning in the monkey vestibulo-ocular reflex. Science. 1984. July 6;225(4657):74–6. [DOI] [PubMed] [Google Scholar]
- 83.Fernandez C, Goldberg JM, Abend WK. Response to static tilts of peripheral neurons innervating otolith organs of the squirrel monkey. J Neurophysiol. 1972. November;35(6):978–87. [DOI] [PubMed] [Google Scholar]
- 84.Angelaki DE, Shaikh AG, Green AM, Dickman JD. Neurons compute internal models of the physical laws of motion. Nature. 2004. July 29;430(6999):560–4. [DOI] [PubMed] [Google Scholar]
- 85.Shaikh AG, Ghasia FF, Dickman JD, Angelaki DE. Properties of cerebellar fastigial neurons during translation, rotation, and eye movements. J Neurophysiol. 2005. February;93(2):853–63. [DOI] [PubMed] [Google Scholar]
- 86.Shaikh AG, Green AM, Ghasia FF, Newlands SD, Dickman JD, Angelaki DE. Sensory convergence solves a motion ambiguity problem. Curr Biol CB. 2005. September 20;15(18):1657–62. [DOI] [PubMed] [Google Scholar]
- 87.Yakusheva TA, Shaikh AG, Green AM, Blazquez PM, Dickman JD, Angelaki DE. Purkinje cells in posterior cerebellar vermis encode motion in an inertial reference frame. Neuron. 2007. June 21;54(6):973–85. [DOI] [PubMed] [Google Scholar]
- 88.Angelaki DE, Hess BJ. Inertial representation of angular motion in the vestibular system of rhesus monkeys. II. Otolith-controlled transformation that depends on an intact cerebellar nodulus. J Neurophysiol. 1995. May;73(5):1729–51. [DOI] [PubMed] [Google Scholar]
- 89.Sheliga BM, Yakushin SB, Silvers A, Raphan T, Cohen B. Control of Spatial Orientation of the Angular Vestibulo-Ocular Reflex by the Nodulus and Uvula of the Vestibulocerebellum. Ann N Y Acad Sci. 1999. May 1;871(1):94–122. [DOI] [PubMed] [Google Scholar]
- 90.Robinson DA. Adaptive gain control of vestibuloocular reflex by the cerebellum. J Neurophysiol. 1976. September;39(5):954–69. [DOI] [PubMed] [Google Scholar]
- 91.Wearne S, Raphan T, Cohen B. Effects of tilt of the gravito-inertial acceleration vector on the angular vestibuloocular reflex during centrifugation. J Neurophysiol. 1999. May;81(5):2175–90. [DOI] [PubMed] [Google Scholar]
- 92.Robinson DA. The use of matrices in analyzing the three-dimensional behavior of the vestibulo-ocular reflex. Biol Cybern. 1982. December 1;46(1):53–66. [DOI] [PubMed] [Google Scholar]
- 93.Raphan T, Matsuo V, Cohen B. Velocity storage in the vestibulo-ocular reflex arc (VOR). Exp Brain Res. 1979. April 1;35(2):229–48. [DOI] [PubMed] [Google Scholar]
- 94.Ramat S, Leigh RJ, Zee DS, Optican LM. What clinical disorders tell us about the neural control of saccadic eye movements. Brain J Neurol. 2007. January;130(Pt 1):10–35. [DOI] [PubMed] [Google Scholar]