Fig. 1.
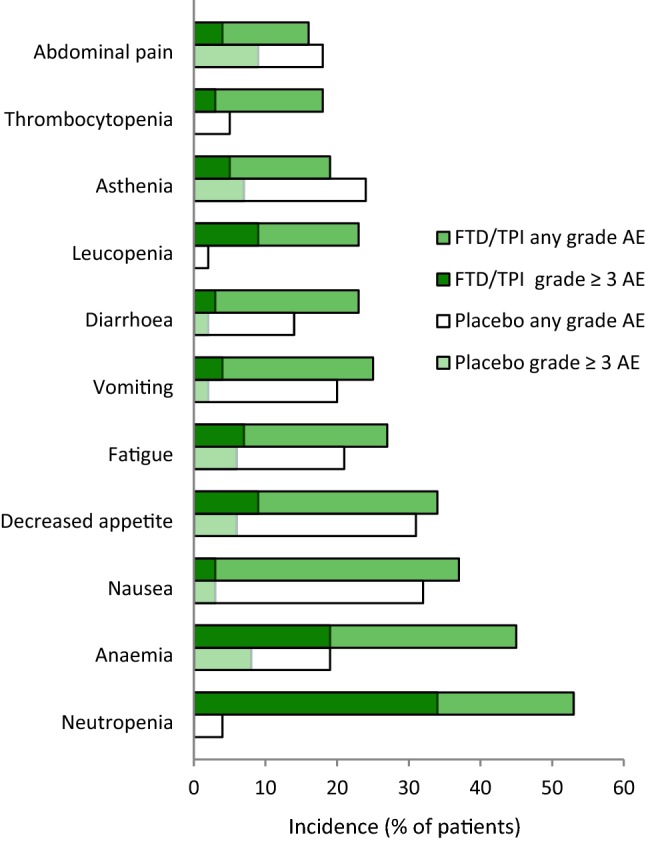
Most common (incidence ≥ 15% in the active treatment group) adverse events of any grade or cause in patients with metastatic gastric cancer who received trifluridine/tipiracil (n = 335) or placebo (n = 168) in the phase III TAGS trial [16]. No grade ≥ 3 thrombocytopenia, leucopenia or neutropenia was reported in the placebo group. AE adverse event, FTD/TPI trifluridine/tipiracil
