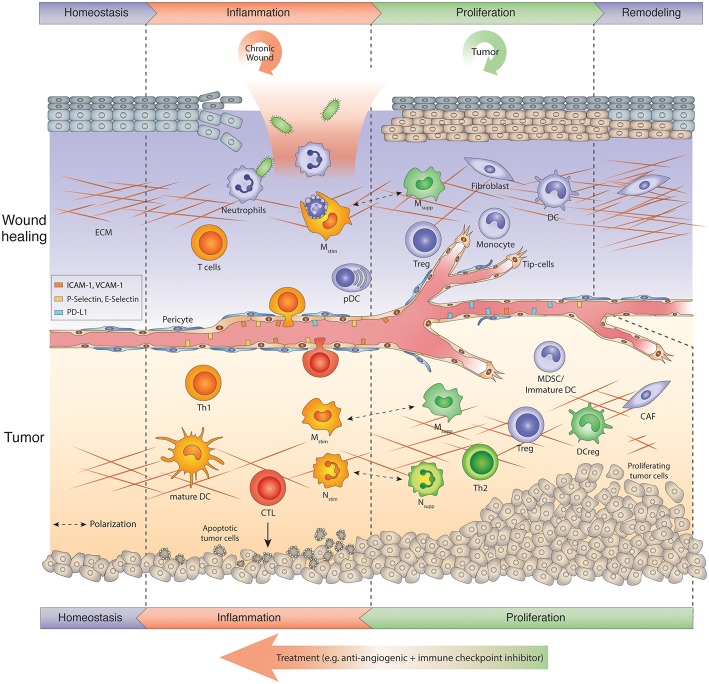
Figure 1.
Tumors hijack the wound repair program: Chronic wound vs. tumor wound. Usually wound healing is manifested in several sequential steps after injury referred to as inflammation, proliferation-resolution, and remodeling phase. Immune cells are key regulators in the wound repair program. In the inflammation phase, neutrophils kill microbes and macrophages phagocytose apoptosing neutrophils, while skin-resident or infiltrating T cells produce IL-17, IL-22, and TNF α to amplify the host defense response. During the proliferation phase, macrophages switch to an anti-inflammatory phenotype (Msupp). Msupp macrophages, Nsupp neutrophils (or tumor-associated neutrophil, TANs), Tregs and other immunosuppressive cells may help to attenuate the inflammation response and facilitate resolution and tissue remodeling. Chronic wounds get trapped in the inflammation phase, exacerbate inflammation and thus, hinder tissue repair. Tumors, on the other hand, hijack the proliferative/remodeling program and provide signals that create a continuous angiogenic and immunosuppressive environment enabling tumors to grow and escape immune surveillance. Therefore, tumors remain in the proliferative phase upon the onset of angiogenesis. Antiangiogenic immunotherapies induce an inflammation program in tumors that reawakens and boosts an anti-tumor response. The ultimate goal is to create a homeostatic situation in which tumor cells are eliminated and a normal tissue architecture is achieved. CAF, Cancer-associated fibroblast; CTL, cytotoxic T lymphocyte; DC, dendritic cell; DCreg, regulatory DC; ECM, extracellular matrix; MDSC, myeloid-derived suppressor cells; Mono, monocyte; Mstim, immunostimulatory macrophage (M1-like); Msupp, immunosuppressive macrophage (M2-like); Nstim, immunostimulatory neutrophil (N1-like); Nsupp, immunosuppressive neutrophil (TAN); pDC, plasmacytoid DC; Th, T helper cell; Treg, regulatory T cell.