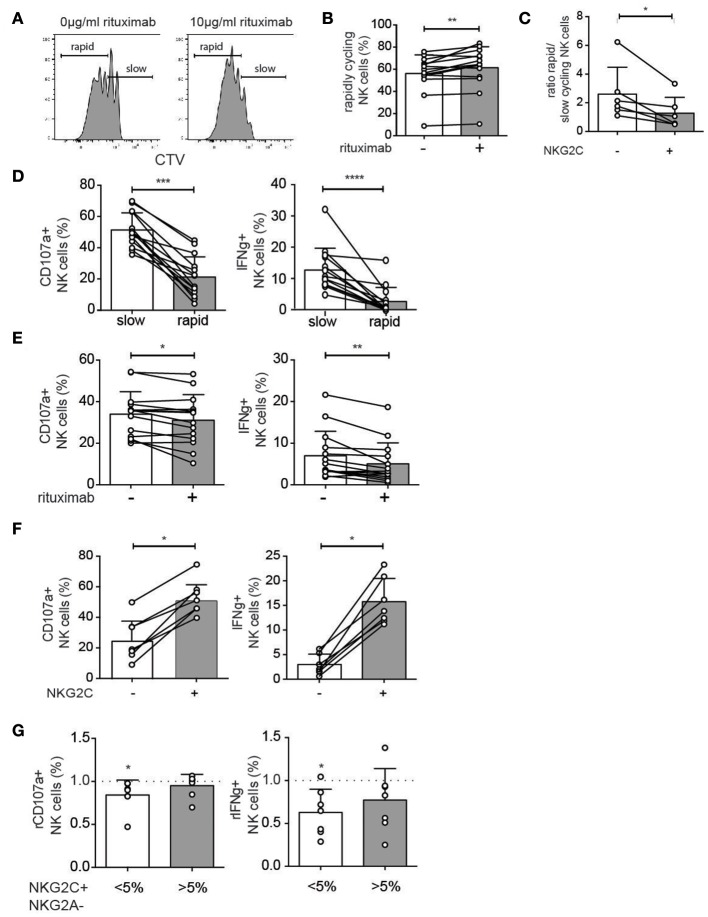
Figure 5.
In vitro model for altered NK cell functions upon rituximab pre-treatment. (A,B) NK cells from CMV-seropositive healthy donors were cultured for 6 days with low-dose IL-15 (1 ng/ml) in the presence or absence of rituximab (10 μg/ml) for the first 48 h. The percentage of rapidly cycling NK cells (at least 2 divisions) was analyzed after 6 days of IL-15 culture with (+) or without (–) rituximab pretreatment. (C) The ratio of rapid versus slowly cycling NK cells as defined in panel A, stratified based on expression of NKG2C. (D) Degranulation (CD107a) and cytokine production (IFNγ) after stimulation with rituximab-coated 721.221 cells in rapidly and slowly cycling NK cells. (E) Degranulation (CD107a) and cytokine production (IFNγ) after stimulation with rituximab-coated 721.221 cells in bulk NK cells with (+) or without (–) rituximab pretreatment in vitro. (F) Degranulation (CD107a) and cytokine production (IFNγ) after stimulation with rituximab-coated 721.221 cells in NKG2C+ and NKG2C- NK cells. (G) Healthy donors were divided into those with (>5%) or without (<5%) a significant expansion of NKG2C+ NK cells. The relative degranulation (rCD107a) and relative IFNγ production (rIFNγ) in response to ADCC stimulation for cells with or without pretreatment with rituximab. Statistical significance between paired samples was calculated using a Wilcoxon test and indicated on bars, whereas a Wilcoxon singed-rank test was used to calculate the statistical significance against a fixed value. Non-paired samples were analyzed by a Mann-Whitney t-test (*p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001).