Abstract
Research objectives
It is widely accepted that mild traumatic brain injury (mTBI) causes injury to the white matter, but the extent of gray matter (GM) damage in mTBI is less clear.
Methods
We tested 26 civilian healthy controls and 14 civilian adult subacute-chronic mTBI patients using quantitative features of MRI-based Gradient Echo Plural Contrast Imaging (GEPCI) technique. GEPCI data were reconstructed using previously developed algorithms allowing the separation of R2t*, a cellular-specific part of gradient echo MRI relaxation rate constant, from global R2* affected by BOLD effect and background gradients.
Results
Single-subject voxel-wise analysis (comparing each mTBI patient to the sample of 26 control subjects) revealed GM abnormalities that were not visible on standard MRI images (T1w and T2w). Analysis of spatial overlap for voxels with low R2t* revealed tissue abnormalities in multiple GM regions, especially in the frontal and temporal regions, that are frequently damaged after mTBI. The left posterior insula was the region with abnormalities found in the highest proportion (50%) of mTBI patients.
Conclusions
Our data suggest that GEPCI quantitative R2t* metric has potential to detect abnormalities in GM cellular integrity in individual TBI patients, including abnormalities that are not detectable by a standard clinical MRI.
Keywords: Traumatic Brain Injury, gray matter, MRI
INTRODUCTION
The Centers for Disease Control and Prevention (CDC) estimate that each year approximately 1.7 million U.S. citizens sustain a traumatic brain injury (TBI).1 TBIs can be classified into mild, moderate, and severe categories, and about 90% of all TBI cases in the USA are classified as mild TBI (mTBI).
Mild TBI is associated with a host of symptoms and signs: headache, confusion, lightheadedness, dizziness, blurred vision or “tired” eyes, tinnitus, fatigue or lethargy, altered sleep patterns, behavioral or mood changes (including post-traumatic stress disorder (PTSD) and depression), and problems with memory, concentration, and attention.2,3 It is unknown why a sizeable percentage of mTBI individuals (~47% of adults, and up to 40% of children)4 continue to manifest symptoms chronically despite that standard structural imaging scans are typically normal.
Consensus is growing that, due to variability of the nature and severity of brain trauma, mTBI-related abnormalities cannot be well described at the group level, therefore emphasizing the need for reliable detection of mTBI-related brain tissue damage at the individual patient level. Many different types of neuroimaging modalities have been used for detecting mTBI (T1 or T2-weighted images, fluid attenuated inversion recovery, diffusion weighted imaging, diffusion tensor imaging (DTI), functional magnetic resonance imaging (fMRI), susceptibility weighted imaging (SWI), magnetic resonance spectroscopy (MRS), magnetization transfer imaging, magnetic resonance elastography, perfusion weighted imaging, arterial spin labeling, MR permeability imaging and MR angiography), but most lack sufficient sensitivity, and no single imaging modality has proven effective for detecting damage in all TBI patients (detailed discussion in5). More sensitive neuroimaging techniques are needed, especially for gray matter abnormalities in TBI. The MRI-based Gradient Echo Plural Contrast Imaging (GEPCI) technique is particularly promising due to its sensitivity to detect impaired integrity of brain cellular structure6–9 as well as high spatial resolution.
Previously we successfully applied the GEPCI technique to studying healthy aging,6 brain pathology in patients with Alzheimer’s disease,7 multiple sclerosis (MS),8,10–15 and psychiatric diseases.16 We have also published several studies to help rigorously validate different aspects of the GEPCI technique.6,17–22 In our recent paper9 by using a multi-modality approach that includes advanced version of GEPCI, volumetric and functional connectivity MRI along with Allan Human Brain Atlas gene expression data we demonstrated that the R2t* metric of GEPCI signal provides a unique genetic and cellular perspective on brain cortical structure. This is a noteworthy finding suggesting that GEPCI metrics can serve as surrogate markers of tissue-cellular integrity identifying brain regions of pathological changes. It is important to underline that GEPCI R2t* surrogate marker of tissue-cellular integrity is substantially different from volumetric MRI surrogate markers. Our data obtained on a cohort of participants with different levels of Alzheimer pathology7 show that GEPCI technique identifies the brain cellular damage before it begins to atrophy, thus suggesting potential for early and more sensitive disease diagnostic.
In this paper, we report initial data using an advanced version13,23,24 of GEPCI technique for detecting microstructural brain changes caused by mTBI. This advanced version of GEPCI technique provides quantitative measurements of the transverse relaxation properties (R2*) of the gradient recalled echo (GRE) MRI signal, and also allows separation of the tissue-cellular-specific relaxation rate constant (R2t*) from the total R2* that also includes contributions from BOLD (blood-oxygen-level dependent) effects.23 The technique incorporates algorithms for correction of adverse background field gradient effects on GEPCI measurements24 and methods for minimizing effects of physiological fluctuations.13 An accuracy analysis of R2t* evaluation from GEPCI measurements was provided in an earlier study.6 The tissue-specific MRI relaxation parameter R2t* depends on the environment of water molecules (the main source of MRI signal), such that higher concentrations of proteins, lipids, and other constituents of cell-building materials of biological tissue-cellular matrix (sources of MR signal relaxation) lead to higher relaxation rate constants. Indeed, in pure water or CSF, the R2t* is about 1 s−1, while in normal brain tissue it is about 15–20 s−1. This substantial dynamic range enhances the ability of R2t* measurements to provide important information on tissue-cellular integrity6,7 and alterations that reflect reduced integrity of brain cellular structure and disease-related tissue damage. Accordingly, the purpose of the present study was to determine whether GEPCI can detect cellular damage in mTBI and thus provide additional diagnostic information which is inaccessible to standard clinical MRI assessments.
METHODS
Subjects
This study was approved by the Institutional Review Board of Washington University School of Medicine. Twelve chronic and two subacute (0.9 and 1.6 months post injury) mTBI civilian patients (10 males and 4 females) were examined. Mean age was 42.9 years (SD = 10.3). TBI patients were diagnosed with mTBI at the Washington University School of Medicine Concussion clinic. Based on the literature,25–28 mTBI was defined as chronic if symptoms persisted for 3 months or more post injury. On average, our cohort of chronic mTBI patients were 28.9 months (SD = 24.71; range 3.1–69.2 months) post-injury (Table I).
TABLE I.
Demographic Data for Each Subject With mTBI and Summary of Radiological Findings
| Subject Number | Age | Gender | Handedness | Education | Race | Cause of TBI | LOC (y/n) | LOC duration | PTA (y/n) | Anterograde PTA duration | GCS | MPI | HISC | PCL_C | CT/MRI brain |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45.4 | F | R | 18 | C | Fall or blow to the head | Yes | Seconds | Yes | 1 hour | 15 | 45.0 | 1 | 20 | Normal CT |
| 2 | 52.2 | M | R | 13 | Ref | MVA | Yes | 2 minutes | Yes | 5 minutes | 15 | 69.2 | 2 | 22 | Normal MRI |
| 3 | 58.1 | M | R | 12 | C | MVA | Yes | 5 minutes | Yes | 1–2 hours | N/A | 55.5 | 2 | 21 | Normal CT |
| 4 | 30.7 | M | A | 13 | AA | MVA | Yes | 1–5 minutes | Yes | 3–5 minutes | N/A | 38.9 | 16 | 69 | Normal CT |
| 5 | 23.9 | F | R | 16 | C | Fall or blow to the head | No | No | Yes | 15 minutes | N/A | 58.6 | 8 | 28 | Normal MRI |
| 6 | 45.3 | M | R | 20 | C | Fall or blow to the head | N/A | N/A | Yes | 9–10 hours | N/A | 39.7 | 1 | 20 | Normal CT |
| 7 | 45.5 | M | L | 14 | C | Fall or blow to the head | Yes | N/A | Yes | N/A | N/A | 13.1 | 3 | 19 | Acute: nondisplaced skull fracture, traumatic SAH; follow-up: resolution of the traumatic subarachnoid blood with no persistent subdural collection. |
| 8 | 30.9 | M | R | 15 | AA | MVA | Yes | N/A | Yes | N/A | N/A | 3.7 | 2 | 25 | N/A |
| 9 | 47.5 | F | N/A | 18 | C | Fall or blow to the head | N/A | N/A | Yes | 5–30 minutess | 15 | 8.1 | 9 | 34 | Facial trauma with orbital wall, maxillary sinus fracture |
| 10 | 41.0 | M | R | 12 | AA | N/A | N/A | N/A | N/A | N/A | N/A | 8.6 | 0 | 17 | N/A |
| 11 | 51.1 | M | R | 13 | AA | Fall or blow to the head | Yes | N/A | Yes | 1–24 hours | 15 | 1.6 | 3 | 25 | Right SAH |
| 12 | 49.7 | F | R | 12 | AA | MVA | Yes | Minutes | Yes | 5–30 minutes | 15 | 0.9 | 11 | 64 | Normal CT |
| 13 | 50.2 | M | R | 14 | C | MVA | No | No | No | No | 15 | 3.7 | 2 | 36 | Normal CT |
| 14 | 28.8 | M | R | 18 | C | Fall or blow to the head | No | No | No | No | N/A | 3.1 | 2 | 17 | Normal CT |
MPI, months post injury; GCS, Glasgow Coma Scale; MVA, motor vehicle accident; N/A, not available; PTA, post-traumatic amnesia; LOC, loss of consciousness; L, left handed; R, right handed; A, ambidextrous; M, male; F, female; U, unknown; AA, African-American; C, Caucasian; A, Asian; Ref, refused; HISC, number of symptoms on HISC 1–20 scale that are appeared or become worse after mTBI; PCL-C, score on PTSD CheckList – Civilian Version; CT, computed tomography; SAH, subarachnoid hemorrhage. Age and education is in years.
The inclusion criteria were: isolated TBI with or without loss of consciousness (LOC) between 3 months to 6 years (for chronic patients) prior to testing, any length of post-traumatic amnesia (PTA), Glasgow Coma Scale (GCS) of 13–15 at time of injury, age 18–60. Exclusion criteria for mTBI patients were: other neurological or pre-morbid psychiatric disorders (including ADHD and seizure disorder), alcohol/substance abuse, gross visual (worse than 20/30 corrected) or hearing problems, metal objects in body not proven to be safe for 3 T MRI, pregnancy and severe claustrophobia. Informed consent was obtained in accordance with procedures approved by the local human studies committees. Twenty six civilian control subjects, including 9 males and 17 females, were also recruited as controls. Mean age was 45.3 years (SD = 13.8). mTBI patients and controls were not significantly (Independent samples Mann–Whitney U test p = 0.664) different in age, although they were not matched by race, gender or socioeconomic status (Tables I and II).
TABLE II.
Demographic Data for Each Control Subject
| Subject Number | Age | Gender | Handedness | Education | Race |
|---|---|---|---|---|---|
| 1 | 58 | F | R | PHD | C |
| 2 | 62 | F | R | BS | C |
| 3 | 36 | M | R | PHD | AA |
| 4 | 34 | F | L | MS | C |
| 5 | 38 | M | R | BS | C |
| 6 | 23 | M | R | MS | C |
| 7 | 47 | F | R | BS | C |
| 8 | 57 | F | R | PHD | C |
| 9 | 75 | F | R | N/A | C |
| 10 | 66 | M | R | PHD | C |
| 11 | 28 | F | R | BS | C |
| 12 | 50 | F | R | HS | C |
| 13 | 52 | F | R | HS | C |
| 14 | 52 | F | R | HS | C |
| 15 | 23 | F | R | BS | C |
| 16 | 42 | F | R | MS | C |
| 17 | 52 | F | R | HS | C |
| 18 | 43 | U | R | PHD | N/A |
| 19 | 45 | F | R | MS | C |
| 20 | 62 | F | R | BS | C |
| 21 | 39 | M | R | BS | C |
| 22 | 47 | F | R | BS | AA |
| 23 | 61 | F | R | N/A | C |
| 24 | 42 | M | R | PHD | C |
| 25 | 26 | M | R | PHD | A |
| 26 | 29 | M | R | PHD | A |
L, left handed; R, right handed; M, male; F, female; U, unknown; AA, African-American; C, Caucasian; A, Asian. Education field lists the highest degree (HS, high school graduate; BS, bachelor’s degree; MS, master’s degree; PHD, doctoral degree). N/A, not available.
Neuropsychological Testing for mTBI Patients
Post-concussive symptoms were measured using the Head Injury Symptom Checklist (HISC).29 PTSD symptoms were measured by PTSD CheckList – Civilian Version (PCL_C).
MRI Data Acquisition
Subjects were scanned in a 3 T Trio MRI scanner (Siemens, Erlangen, Germany) with a 32-channel phased-array head coil (except for 2 recent mTBI patients that were scanned after Trio was upgraded to Prisma scanner). A 3D gradient recalled echo (GRE) MRI sequence with multiple gradient echoes was used for data acquisition. Sequence parameters were: resolution 1 × 1 × 2 mm3 (read, phase, slab), FOV 256 mm × 192 mm, repetition time TR = 50 ms, flip angle 30°, 10 gradient echoes with first echo time TE1 = 4 ms, echo spacing ∆TE = 4 ms. Additional phase stabilization echo (the navigator data) was collected for each line in k-space to correct for image artifacts due to physiological fluctuations.13
Standard clinical MP-RAGE images with voxel size: 1 × 1 × 1 mm3 (0.8 × 0.8 × 0.8 mm3 for the two patients scanned on Prisma) were also collected for segmentation and atlas transformation purposes. For mTBI patients we also collected T2-weighted images with voxel size: 1 × 1 × 1 mm3 (0.8 × 0.8 × 0.8 mm3 for two patients scanned on Prisma).
Data Analysis and Images Generation
Image processing was done in MATLAB (The MathWorks, Inc.). 3D GEPCI images were obtained after correcting the k-space data for physiological fluctuations.13 To remove the initial phase incoherence among the MR signals from different RF channels and achieve an optimal signal-to-noise ratio for parameter estimation, we use the following equation to combine the data of all 32 RF channels into a single data set S:12,30
| 1 |
where TE is the gradient echo time, the sum is taken over all 32 RF channels, and m (m’) is the RF channel number, denotes complex conjugate of , are weighting parameters and are noise amplitudes (r.m.s.). Index n corresponds to the voxel position (n = x,y,z). 3D spatial Hanning filter was then applied to the data in the image domain.
The combined data were analyzed on a voxel-by-voxel basis using a theoretical model:23
| 2 |
where is the tissue-cellular-specific transverse relaxation rate constant, Δf is the frequency shift (dependent on tissue structure and also macroscopic magnetic field created mostly by tissue/air interfaces), and function F(TE) accounts for the contribution of macroscopic magnetic field inhomogeneities to the GRE signal. In this paper, we use a voxel spread function (VSF) method24 for calculating F(TE). The Function FBOLD(TE) describes GRE signal decay due to the presence of blood vessel network with deoxygenated blood, as described previously:23
where is the deoxygenated cerebral blood volume fraction (dCBV), and is the characteristic frequency determined by the susceptibility difference between deoxygenated blood and surrounding tissue. Function fs was previously defined.23
GEPCI T1-weighted (T1W) images (parameter S0 in Equation [2]) were co-registered to MP-RAGE images. MP-RAGE images were then transformed to an atlas space representative target using a 12-parameter affine transformation31 and resampled to 1 × 1 × 1 mm3 voxel size. The corresponding transformation matrices were then applied to GEPCI R2t* maps that are naturally co-registered with GEPCI T1W images.
Statistical analyses
For voxel-wise single-subject analysis, we used the previously published method.32 All images were transformed to a standard Talairach atlas space. For each mTBI patient (n = 14), R2t* images were compared to R2t* images from the healthy controls (n = 26) by using Student t-test, similar to a prior report32 and masked by voxels that were inside brain for each subject. Each uncorrected z-map (t-map was converted into z-map) was thresholded at z < −2 (negative value means patient < controls) and converted into binary mask (voxels with z < −2 were assigned value 1 while all other voxels were assigned value 0). Next we computed average of those binary masks and each voxel’s value was representing the degree of overlap (where a value of 0.5 means that particular voxel was identified as abnormal (with z < −2) in 7 of the 14 mTBI subjects). Next we counted number of clusters (continuous voxels with specific value and size (≥27 mm3) on the image representing average of those binary masks) by using automated algorithm.
RESULTS
Comparing GEPCI Images With Standard Clinical Images
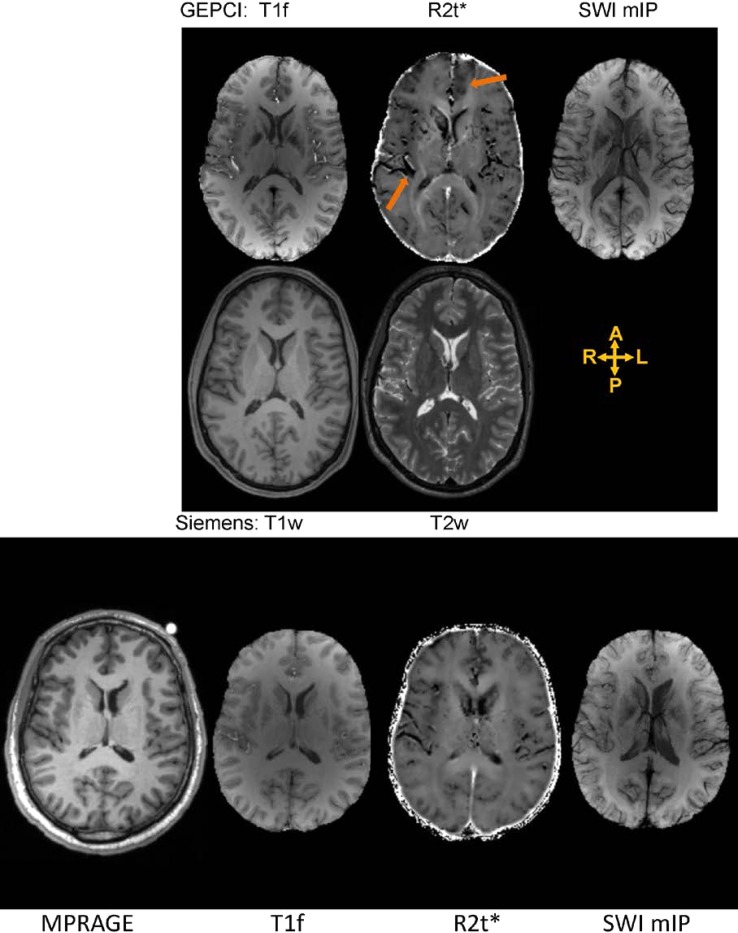
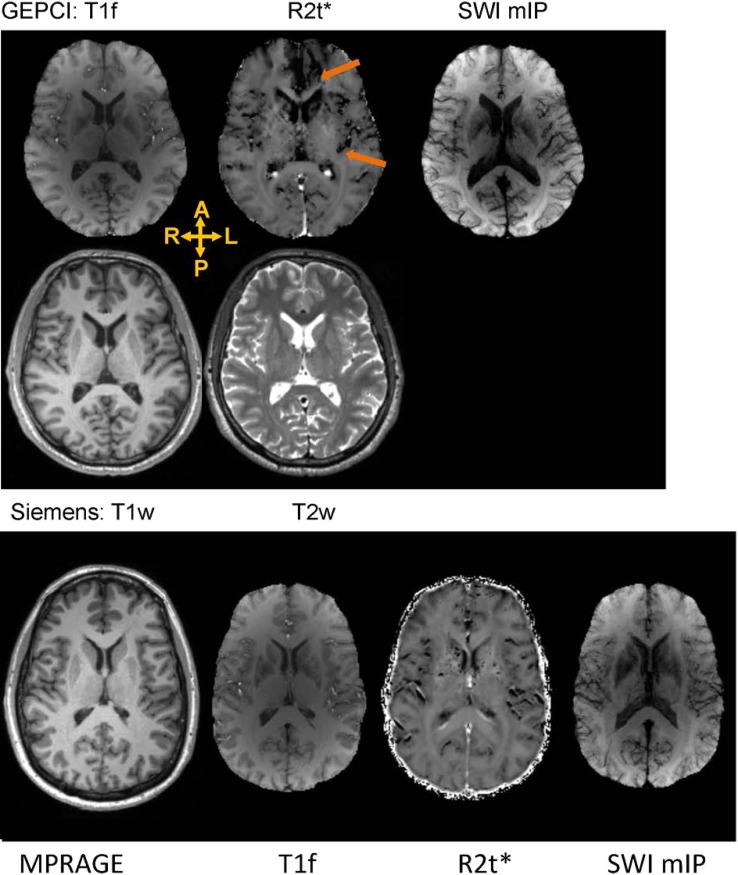
Figures 1 and 2 show GEPCI alongside standard anatomical MR images for two mTBI patients and two control subjects of the same gender and similar age. Both mTBI subjects displayed reduction of R2t* in frontal and insular cortical areas, in comparison to the control subjects. Thus, analysis of images from single mTBI subjects suggests that R2t*can be sensitive to the presence and localization of TBI related abnormalities. Note that all images are in the native space and standard Siemens images were co-registered to GEPCI images (all GEPCI images are internally co-registered, see methods). Top row shows images generated from a single GEPCI scan in mTBI subject: T1f (combination of T1 weighted and frequency map with improved white matter (WM)/gray matter (GM) contrast),12 R2t*6,7 and SWI mIP.12 SWI mIP image represents SWI minimum intensity projection across five slices. Second row shows Siemens standard images (T1w and T2w) displaying similar slice. Third rows on Figures 1 and 2 present images for control subjects (MPRAGE, T1f, R2t*, SWI mIP). Orange arrows point to dark areas showing abnormal contrast on GEPCI R2t* images only.
FIGURE 1.
Top row: Images generated from a single GEPCI scan in a 29-year-old male mTBI subject 3.1 months post-injury. The subject had two HISC symptoms (headaches and memory problems). Images are: T1f (combination of T1 weighted and frequency map with improved over GEPCI T1W image WM/GM contrast), R2t* and SWI. SWI image represent SWI minimum intensity projection (mIP) across five slices. Arrows point to the dark area in the left frontal lobe and right posterior insula on R2t* (image is in radiological orientation). Second row: Siemens sequences (T1w, T2w) displaying similar axial slice. Bottom row: images from control subject (35-year-old male) A: anterior, P: posterior, R: right, L: left.
FIGURE 2.
Top row: Images generated from a GEPCI scan obtained in a 45-year-old male mTBI patient who was 39.7 months post-injury. He had only one HISC symptom of headache. Images are: T1f (combination of T1 weighted and frequency map with improved WM/GM contrast), R2t* and SWI. The SWI image represents SWI minimum intensity projection across 5 slices. The arrows point to a hypointense areas in the left frontal lobe and posterior insula on the R2t* map. Second row: Siemens sequences (T1w, T2w) displaying similar axial slice. Bottom row: images from gender/age matched control subject (43-year-old male). A: anterior, P: posterior, R: right, L: left.
Quantitative R2t* Analysis of Tissue Damage in mTBI Patients
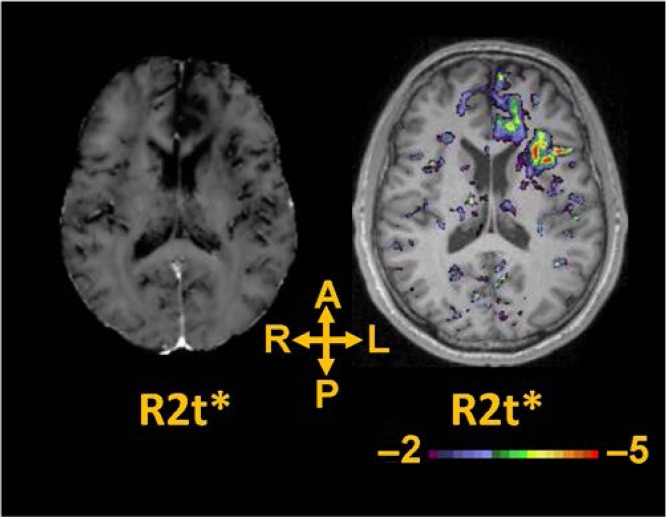
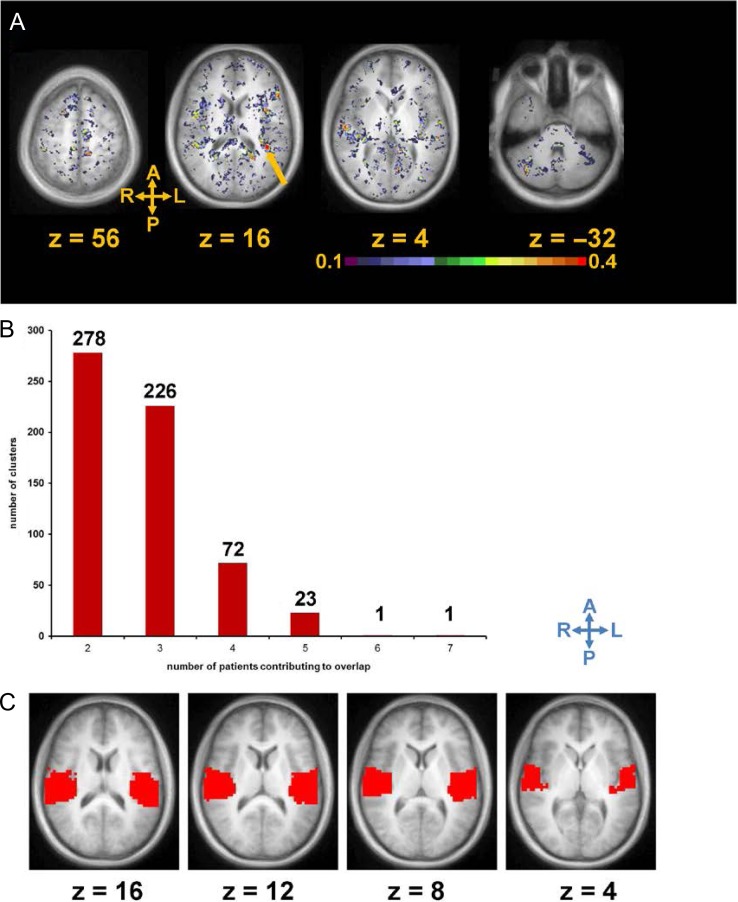
After transforming into standard atlas space, R2t* images from 14 individual mTBI patients were compared to the R2t*images in standard atlas space derived from the healthy control cohort (n = 26). Significantly lower R2t* values relative to healthy controls were noted in many cortical areas of the mTBI patients. Figure 3 presents data from the subject shown on Figure 2. We can see that R2t* image demonstrates significantly lower R2t* values in many cortical areas, although the patient has reported only 1 HISC symptom (headache). While individual mTBI patients showed heterogeneous and multifocal brain abnormalities, we also found regions of abnormal tissue (with reduced R2t*) common to more than one mTBI patient. To summarize single patient abnormalities as well as to identify brain areas that were affected in more than one mTBI patient, for each mTBI patient (n = 14), z-map of R2t* values was thresholded at z < −2 (p < 0.05, uncorrected; negative value means patient < control) and converted into a binary mask (Fig. 4A). For our 14 mTBI subjects, a region common to two would represent 14.29% (2 /14). Clusters of voxels with low R2t* with size of 27 mm3 similar to typical resolution of functional MRI data (3 mm × 3 mm × 3 mm) or greater that were found to be spatially overlapped in at least two mTBI patients (2 of 14 mTBI subjects, or 14.29% or greater commonality) were identified. 278 such clusters were found, with the average cluster size of 503 mm3. As expected, the number of abnormal regions with commonality among mTBI subjects decreased as the number of subjects with overlapping abnormal regions increased.
FIGURE 3.
Images in standard space generated from a single GEPCI scan in the 45-year-old mTBI male subject from Figure 2 (39.7 months post-injury with 1 HISC symptom of headache). Left: R2t* image in a standard space. R2t* image demonstrates significantly lower R2t* values in several cortical areas. Right: z map thresholded at z < −2 (p < 0.05 uncorrected) displayed superimposed on the MPRAGE scan at the same axial level.
FIGURE 4.
Cluster analysis of tissue damage in a cohort of 14 TBI patients displayed with color coding representing the frequency of damage noted in different regions. (A): Averages of 14 binary images in which each subject has been compared voxel by voxel to the entire healthy cohort using R2t*. A value of 0.5 means that particular voxel was significantly lower than controls in 50% of images (in 7 of 14 mTBI patients). The arrow points to a cluster of abnormal voxels with the highest degree of commonality among the mTBI subjects, in left posterior insula. (B) Number of clusters vs. number of patients contributing to overlap. (C) Spatial location of region containing posterior insula selected from 26 functional ROIs defined in previously published rs-fcfMRI analysis.
One region displayed reduced R2t* in 50% of the 14 mTBI patients, and had cluster size of 34 mm3 (Fig. 4B). This area of tissue had the highest degree of overlap across mTBI patients and was located in the left posterior insula (Fig. 4A). The other regions with overlaps to a lesser degree among mTBI subjects included frontal, temporal and parietal areas, as well as the right cerebellum (Fig. 4A). To test for random noise and nonspecific findings, we performed similar analysis among the 26 control subjects by comparing each control subject to the remaining 25 control subjects by t-test. No clusters of common voxels with the size of 27 mm3 or greater were identified in 14.29% or more of the 26 controls.
For our exploratory analysis, we selected a temporal ROI (region of interest), containing posterior insula region with the highest degree of overlap (Fig. 4C), which was selected from 26 functional ROIs defined in a previously published resting-state functional connectivity functional MRI (rs-fcfMRI) analysis.33 Selecting independently defined ROI from rs-fcfMRI allowed us to test overlap of abnormal tissue in previously described important functional hub. In previous analysis, we counted number of clusters on single image, representing the degree of overlap of abnormal tissue in mTBI patients. For our exploratory analysis, we computed number of clusters for each mTBI patient and control (each uncorrected z-map was thresholded at z < −2 and converted into binary mask (see methods)). mTBI patients had significantly higher (compare to controls) number of clusters of abnormal tissue (with z < −2 and size of 100 mm3 or greater) in this ROI (independent samples Mann–Whitney U test p = 0.0056, 95% confidence interval: from 0.12 to 0.42), suggesting that using this ROI (Fig. 4C) may benefit future mTBI studies. R2t* signal extracted from gray matter inside this ROI has demonstrated a tendency for negative correlation with PTSD symptoms measured by PCL_C (Spearman’s ρ = −0.31, p = 0.28), meaning that reduced R2t* may be associated with increased severity of PTSD, but it has not reached the significance level (probably due to the small sample size). We also performed voxel-wise independent samples t-test, comparing the means of R2t* signal for control and mTBI group. Small number of clusters consisting of voxels with reduced R2t* in mTBI group, that survived multiple comparisons correction was in/around insula area, similar to the analysis described above.
DISCUSSION
TBI is a common problem among military personnel and civilians alike. Not only can TBI cause chronic and sometimes incapacitating symptoms, it may also predispose the patient with TBI to Alzheimer-type dementia later in life. Therefore, it is important to identify those TBI patients with substantial underlying damage.
Moderate or severe TBI can be detected by imaging using several methods. Regional brain atrophy is one such method, but atrophy is not common in those with only mild TBI. In fact, mTBI is typically invisible using standard imaging techniques, although more advanced techniques may detect it. The main underlying pathological substrate in mTBI is axonal damage and disruption, which are located largely in the WM.34–37 Currently, WM damage due to TBI is especially suited for evaluation by DTI. While most researchers recognize that TBI produces diffuse injury in the brain WM (including multifocal axonal injury),34–37 GM damage gets less attention in MRI studies, perhaps due to difficulties detecting it by neuroimaging in vivo.
Yet, histological studies on postmortem brains indicate that chronic traumatic encephalopathy (CTE; considered to be a result of repeated concussions and/or traumatic brain injuries) affects both GM and WM.38–40 MRS studies in subjects with TBI demonstrated decreased brain concentrations of N-acetylaspartate (a possible marker of neuronal density and viability), and lower levels of gray matter glutamate-glutamine signal.41,42 Here we demonstrate that the novel GEPCI-derived tissue-specific R2t* metric can detect GM tissue damage in individual patients with mTBI, identifying compromised cellular integrity in GM that was not visible on standard clinical MRI.
The frontal and temporal regions of the brain are established as being particularly vulnerable to TBI,43–45 but little is known about long-term effect of those injuries. Our results showed that in a high proportion of our small cohort of mild TBI patients, significantly decreased R2t* values were found in the insular cortex, a GM region deep within the temporal lobe.
Our finding that posterior insula contained tissue of reduced R2t* in a sizeable proportion of chronic mTBI subjects in our cohort may suggest not only that this area is vulnerable to damage from brain trauma but that is an important functional center.46 Damage to the insula is responsible for many of the persistent symptoms after mTBI.
Our results are complementary to previous findings of decreased insular cortex thickness seen after TBI.47,48 In prior studies, insular atrophy correlated with worse scores of PTSD, depression, and post-concussive symptoms.47,48 Some recent studies also point to the insula as being important functional hub, with damage to it leading to a variety of deficits.46,49 Abnormalities of insula cortex function may contribute to anxiety disorders, emotion dysregulation, addiction,50 and disorders of motor control, empathy, language, social emotions and attention reorienting51 that are not dissimilar to the wide range of symptoms that can occur after mTBI.2,3
Since we have not performed histological analysis, we can only hypothesize about the physiological basis of the altered R2t* values that provide integrated characteristic of tissue-cellular damage after mTBI. Multiple processes can lead to compromised GM and WM cellular density/integrity that might be reflected in reduced R2t* values.6,7 Reduced R2t* values in WM may suggest axonal damage and/or tissue inflammation. This explanation would be in keeping with our previous findings in MS.6–8,10–12,14,52 TBI at the acute stage is also associated with metabolic changes that involve disturbances of potassium, sodium, and calcium ion balances, as well as hyperglycolysis, glutamate alterations, and even apoptosis and can lead to the tissue damage.53 An altered level of amyloid-beta peptide54 was also reported after TBI. Compared to many other disorders, TBI patients are especially heterogeneous with multifocal locations and a wide range of severity of tissue damage. Thus, TBI is less suited for group-based analyses. Hence, there is a great need for imaging modalities and methods to assess damaged tissue in individual TBI patients. Herein we tested a novel GEPCI technique on several mTBI patients and evaluated its potential for identifying abnormal tissue (especially in GM), with promising results. The GEPCI R2t* technique may be especially helpful for implementation in future studies aimed at the individual patient level.
The main limitation of this study is the small sample size with wide range of chronicity, so the findings will require replication and validation in separate and larger independent TBI patient samples. Our control subjects were not matched by race, gender or socioeconomic status. Since we have not tested our mTBI patients before mTBI injury, we cannot unambiguously prove that the observed abnormalities are indeed the result of mTBI injury.
Future studies will be needed to determine correlations between reduction in R2t* and the neuropsychiatric sequelae of TBI. Future human postmortem studies will be necessary to define the histological basis of the abnormalities detected by reduced GEPCI-derived R2t*. Animal studies may also be helpful to better understand the pathological basis of altered R2t*values after TBI.
CONCLUSIONS
In our pilot study on mTBI patients, we demonstrated that GEPCI-derived tissue-specific R2t* metric detected cortical GM tissue abnormalities in subjects with mTBI. Importantly, R2t* values extracted from GEPCI imaging detected compromised GM cellular integrity that was not visible on standard clinical MRI. This imaging technique provides information supplemental to the information on WM damage detected by DTI and other modalities, but in the absence of pre-injury scans, detailed radiological-pathological correlations, or other corroborating data, we cannot determine with certainty whether the changes seen represent effects of TBI. Since mTBI in civilian and military population have many similarities, the GEPCI technique has a potential for detecting mTBI-related brain abnormalities in military personnel.
Previous Presentations
Presented as an abstract at the 2017 Military Health System Research Symposium; August 27–30, 2017, Kissimmee, FL (MHSRS abstract number: MHSRS-17-0134). Presented as an abstract at the Neuroscience 2017 (Society for Neuroscience), November 11–15, 2017, Washington, DC.
Funding
This work was supported by James S. McDonnell Foundation (grant for the Attention Dynamics Consortium in Traumatic Brain Injury), National Institutes of Health (award numbers: R01NS095741, R01HD083614, R21DA038834, R01AG054513) and National Multiple Sclerosis Society (grant RG 4463A18). Scanning services were partially supported by the Mallinckrodt Institute of Radiology (MIR) Facilities Pilot Study Fund (award MIR 14-021). This supplement was sponsored by the Office of the Secretary of Defense for Health Affairs.
References
- 1. Faul M, Xu L, Wald MM, Coronado VG: Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Atlanta (GA), Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, 2010. [Google Scholar]
- 2. Chen AJ, D’Esposito M: Traumatic brain injury: from bench to bedside [corrected] to society. Neuron 2010; 66(1): 11–4. [DOI] [PubMed] [Google Scholar]
- 3. McAllister TW: Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci 2011; 13(3): 287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crooks CY, Zumsteg JM, Bell KR: Traumatic brain injury: a review of practice management and recent advances. Phys Med Rehabil Clin N Am 2007; 18(4): 681–710. vi. [DOI] [PubMed] [Google Scholar]
- 5. Amyot F, Arciniegas DB, Brazaitis MP, et al. : A review of the effectiveness of neuroimaging modalities for the detection of traumatic brain injury. J Neurotrauma 2015; 32(22): 1693–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao Y, Wen J, Cross AH, Yablonskiy DA: On the relationship between cellular and hemodynamic properties of the human brain cortex throughout adult lifespan. Neuroimage 2016; 133: 417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao Y, Raichle ME, Wen J, et al. : In vivo detection of microstructural correlates of brain pathology in preclinical and early Alzheimer Disease with magnetic resonance imaging. Neuroimage 2017; 148: 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wen J, Yablonskiy DA, Salter A, Cross AH: Limbic system damage in MS: MRI assessment and correlations with clinical testing. PLoS One 2017; 12(11): e0187915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wen J, Goyal MS, Astafiev SV, Raichle ME, Yablonskiy DA: Genetically defined cellular correlates of the baseline brain MRI signal. Proc Natl Acad Sci USA 2018; 115(41): E9727–E9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sati P, Cross AH, Luo J, Hildebolt CF, Yablonskiy DA: In vivo quantitative evaluation of brain tissue damage in multiple sclerosis using gradient echo plural contrast imaging technique. Neuroimage 2010; 51(3): 1089–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luo J, Yablonskiy DA, Hildebolt CF, Lancia S, Cross AH: Gradient echo magnetic resonance imaging correlates with clinical measures and allows visualization of veins within multiple sclerosis lesions. Mult Scler 2014; 20(3): 349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luo J, Jagadeesan BD, Cross AH, Yablonskiy DA: Gradient echo plural contrast imaging--signal model and derived contrasts: T2*, T1, phase, SWI, T1f, FST2*and T2*-SWI. Neuroimage 2012; 60(2): 1073–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wen J, Cross AH, Yablonskiy DA: On the role of physiological fluctuations in quantitative gradient echo MRI: implications for GEPCI, QSM, and SWI. Magn Reson Med 2015; 73(1): 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wen J, Yablonskiy DA, Luo J, Lancia S, Hildebolt C, Cross AH: Detection and quantification of regional cortical gray matter damage in multiple sclerosis utilizing gradient echo MRI. Neuroimage Clin 2015; 9: 164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel KR, Luo J, Alvarez E, et al. : Detection of cortical lesions in multiple sclerosis: a new imaging approach. Mult Scler J Exp Transl Clin 2015; 1: 2055217315606465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mamah D, Wen J, Luo J, Ulrich X, Barch DM, Yablonskiy D: Subcomponents of brain T2* relaxation in schizophrenia, bipolar disorder and siblings: a Gradient Echo Plural Contrast Imaging (GEPCI) study. Schizophr Res 2015; 169(1–3): 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yablonskiy DA, Haacke EM: Theory of NMR signal behavior in magnetically inhomogeneous tissues: the static dephasing regime. Magn Reson Med 1994; 32(6): 749–63. [DOI] [PubMed] [Google Scholar]
- 18. Yablonskiy DA: Quantitation of intrinsic magnetic susceptibility-related effects in a tissue matrix. Phantom study. Magn Reson Med 1998; 39(3): 417–28. [DOI] [PubMed] [Google Scholar]
- 19. He X, Zhu M, Yablonskiy DA: Validation of oxygen extraction fraction measurement by qBOLD technique. Magn Reson Med 2008; 60(4): 882–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spees WM, Yablonskiy DA, Oswood MC, Ackerman JJ: Water proton MR properties of human blood at 1.5 Tesla: magnetic susceptibility, T(1), T(2), T*(2), and non-Lorentzian signal behavior. Magn Reson Med 2001; 45(4): 533–42. [DOI] [PubMed] [Google Scholar]
- 21. Dickson JD, Ash TW, Williams GB, Sukstanskii AL, Ansorge RE, Yablonskiy DA: Quantitative phenomenological model of the BOLD contrast mechanism. J Magn Reson 2011; 212(1): 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang X, Sukstanskii AL, Yablonskiy DA: Optimization strategies for evaluation of brain hemodynamic parameters with qBOLD technique. Magn Reson Med 2013; 69(4): 1034–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ulrich X, Yablonskiy DA: Separation of cellular and BOLD contributions to T2* signal relaxation. Magn Reson Med 2016; 75(2): 606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yablonskiy DA, Sukstanskii AL, Luo J, Wang X: Voxel spread function method for correction of magnetic field inhomogeneity effects in quantitative gradient-echo-based MRI. Magn Reson Med 2013; 70(5): 1283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kovesdi E, Luckl J, Bukovics P, et al. : Update on protein biomarkers in traumatic brain injury with emphasis on clinical use in adults and pediatrics. Acta Neurochir (Wien) 2010; 152(1): 1–17. [DOI] [PubMed] [Google Scholar]
- 26. Leddy JJ, Kozlowski K, Donnelly JP, Pendergast DR, Epstein LH, Willer B: A preliminary study of subsymptom threshold exercise training for refractory post-concussion syndrome. Clin J Sport Med 2010; 20(1): 21–7. [DOI] [PubMed] [Google Scholar]
- 27. McCrea M, Iverson GL, McAllister TW, et al. : An integrated review of recovery after mild traumatic brain injury (MTBI): implications for clinical management. Clin Neuropsychol 2009; 23(8): 1368–90. [DOI] [PubMed] [Google Scholar]
- 28. Yuh EL, Mukherjee P, Lingsma HF, et al. : Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann Neurol 2013; 73(2): 224–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McLean A Jr., Dikmen S, Temkin N, Wyler AR, Gale JL: Psychosocial functioning at 1 month after head injury. Neurosurgery 1984; 14(4): 393–9. [DOI] [PubMed] [Google Scholar]
- 30. Quirk JD, Sukstanskii AL, Bretthorst GL, Yablonskiy DA: Optimal decay rate constant estimates from phased array data utilizing joint Bayesian analysis. J Magn Reson 2009; 198(1): 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rowland DJ, Garbow JR, Laforest R, Snyder AZ: Registration of [18F]FDG microPET and small-animal MRI. Nucl Med Biol 2005; 32(6): 567–72. [DOI] [PubMed] [Google Scholar]
- 32. Chang DJ, Zubal IG, Gottschalk C, et al. : Comparison of statistical parametric mapping and SPECT difference imaging in patients with temporal lobe epilepsy. Epilepsia 2002; 43(1): 68–74. [DOI] [PubMed] [Google Scholar]
- 33. Power JD, Cohen AL, Nelson SM, et al. : Functional network organization of the human brain. Neuron 2011; 72(4): 665–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gennarelli TA: Future directions in brain injury research. Prog Neurol Surg 2014; 28: 243–50. [DOI] [PubMed] [Google Scholar]
- 35. Adams JH, Jennett B, Murray LS, Teasdale GM, Gennarelli TA, Graham DI: Neuropathological findings in disabled survivors of a head injury. J Neurotrauma 2011; 28(5): 701–9. [DOI] [PubMed] [Google Scholar]
- 36. Siman R, Giovannone N, Hanten G, et al. : Evidence that the blood biomarker SNTF predicts brain imaging changes and persistent cognitive dysfunction in mild TBI patients. Front Neurol 2013; 4: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smith DH, Johnson VE, Stewart W: Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol 2013; 9(4): 211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McKee AC, Stein TD, Kiernan PT, Alvarez VE: The neuropathology of chronic traumatic encephalopathy. Brain Pathol 2015; 25(3): 350–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Daneshvar DH, Goldstein LE, Kiernan PT, Stein TD, McKee AC: Post-traumatic neurodegeneration and chronic traumatic encephalopathy. Mol Cell Neurosci 2015; 66(Pt B): 81–90. [DOI] [PubMed] [Google Scholar]
- 40. Kriegel J, Papadopoulos Z, McKee AC: Chronic traumatic encephalopathy: is latency in symptom onset explained by tau propagation? Cold Spring Harb Perspect Med 2018; 8:a024059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gasparovic C, Yeo R, Mannell M, et al. : Neurometabolite concentrations in gray and white matter in mild traumatic brain injury: an 1H-magnetic resonance spectroscopy study. J Neurotrauma 2009; 26(10): 1635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Widerstrom-Noga E, Govind V, Adcock JP, Levin BE, Maudsley AA: Subacute pain after traumatic brain injury is associated with lower insular N-acetylaspartate concentrations. J Neurotrauma 2016; 33(14): 1380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goldstein LE, Fisher AM, Tagge CA, et al. : Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 2012; 4(134): 134ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meier TB, Bellgowan PS, Bergamino M, Ling JM, Mayer AR: Thinner cortex in collegiate football players with, but not without, a self-reported history of concussion. J Neurotrauma 2016; 33(4): 330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Singh R, Meier TB, Kuplicki R, et al. : Relationship of collegiate football experience and concussion with hippocampal volume and cognitive outcomes. JAMA 2014; 311(18): 1883–8. [DOI] [PubMed] [Google Scholar]
- 46. de Pasquale F, Corbetta M, Betti V, Della Penna S: Cortical cores in network dynamics. Neuroimage 2018; 180(Pt B): 370–382. [DOI] [PubMed] [Google Scholar]
- 47. Michael AP, Stout J, Roskos PT, et al. : Evaluation of cortical thickness after traumatic brain injury in military veterans. J Neurotrauma 2015; 32(22): 1751–8. [DOI] [PubMed] [Google Scholar]
- 48. Wolf EJ, Sadeh N, Leritz EC, et al. : Posttraumatic stress disorder as a catalyst for the association between metabolic syndrome and reduced cortical thickness. Biol Psychiatry 2016; 80(5): 363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Corbetta M, Ramsey L, Callejas A, et al. : Common behavioral clusters and subcortical anatomy in stroke. Neuron 2015; 85(5): 927–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Contreras M, Ceric F, Torrealba F: Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science 2007; 318(5850): 655–8. [DOI] [PubMed] [Google Scholar]
- 51. Corbetta M, Shulman GL: Spatial neglect and attention networks. Annu Rev Neurosci 2011; 34: 569–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yablonskiy DA, Luo J, Sukstanskii AL, Iyer A, Cross AH: Biophysical mechanisms of MRI signal frequency contrast in multiple sclerosis. Proc Natl Acad Sci U S A 2012; 109(35): 14212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Giza CC, Hovda DA: The new neurometabolic cascade of concussion. Neurosurgery 2014; 75(Suppl 4): S24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brody DL, Magnoni S, Schwetye KE, et al. : Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science 2008; 321(5893): 1221–4. [DOI] [PMC free article] [PubMed] [Google Scholar]