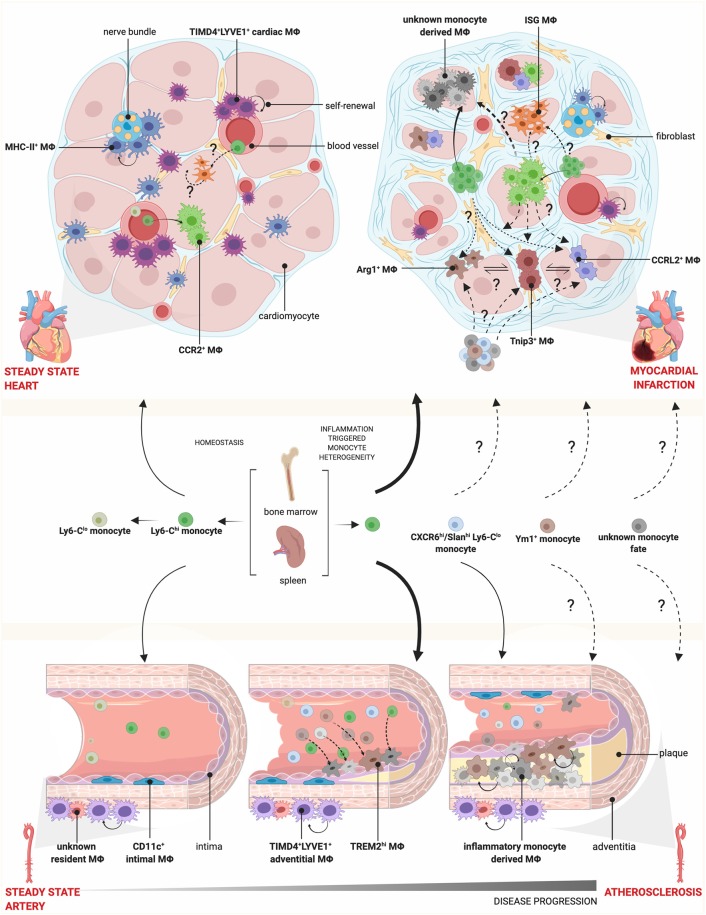
Figure 1.
Monocyte and macrophage heterogeneity in steady state and cardiovascular disease. During homeostasis, Ly6Chi monocytes circulate through blood vessels and infiltrate tissue, where they give rise to CCR2+ MΦs, while Ly6Clo monocytes patrol the vasculature. Cardiac MΦs are further composed of monocyte-independent self-renewing TIMD4+LYVE1+ and MHC-II+ resident MΦs, which localize preferentially near blood vessels and nerve bundles, respectively. During myocardial infarction, there is increased monopoiesis and release of Ly6Chi monocytes from the spleen and bone marrow, which are recruited to the injured heart and give rise to diverse MΦ subsets. Whether these MΦ subsets are a spectrum of activation states or arise via pre-defined monocyte fates, such as Ym1+ or CXCR6hi/Slanhi Ly6Clo monocytes as identified in other disease models, is not known. Conversely, there is a loss of TIMD4+LYVE1+ and MHC-II+ resident MΦs. In the vessels, the intima is lined with CD11c+ MΦs and the adventitia contain TIMD4+LYVE1+ MΦs and other undefined resident MΦ populations. In atherosclerosis, TREM2hi MΦs and inflammatory monocyte-derived MΦs accumulate in the intima, expand via self-renewal and participate in plaque growth. How this fate is defined and the contribution of CXCR6hi/Slanhi Ly6Clo monocytes, found in the circulation of patients correlating with disease severity, is unknown. Mϕ, macrophage.