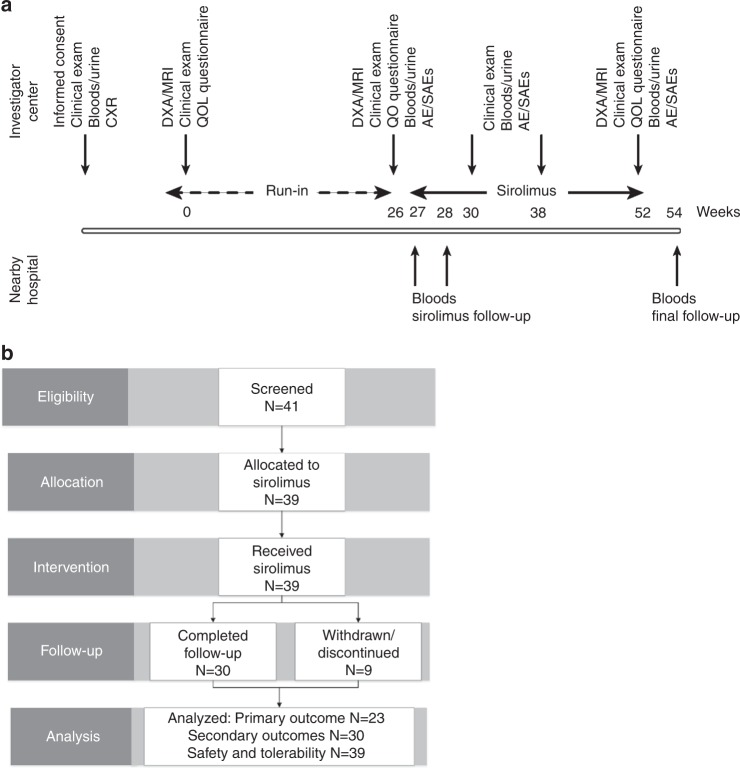
Fig. 1.
CONSORT flow-chart and schematic of nonrandomized open-label pilot study. a Schematic of number of participants assessed for eligibility and excluded or allocated to the study, treated, followed, and analyzed. Of the 39 subjects enrolled, 30 completed 26 weeks of sirolimus therapy, and 23/30 had anatomy that permitted analysis of the primary outcome measure. Safety and tolerability were evaluated in all treated participants. b Overview of study design including schedule of procedures. AE/SAEs adverse events/serious adverse events, CXR chest X-ray, DXA/MRI dual energy X-ray absorptiometry/magnetic resonance imaging, QOL quality of life.