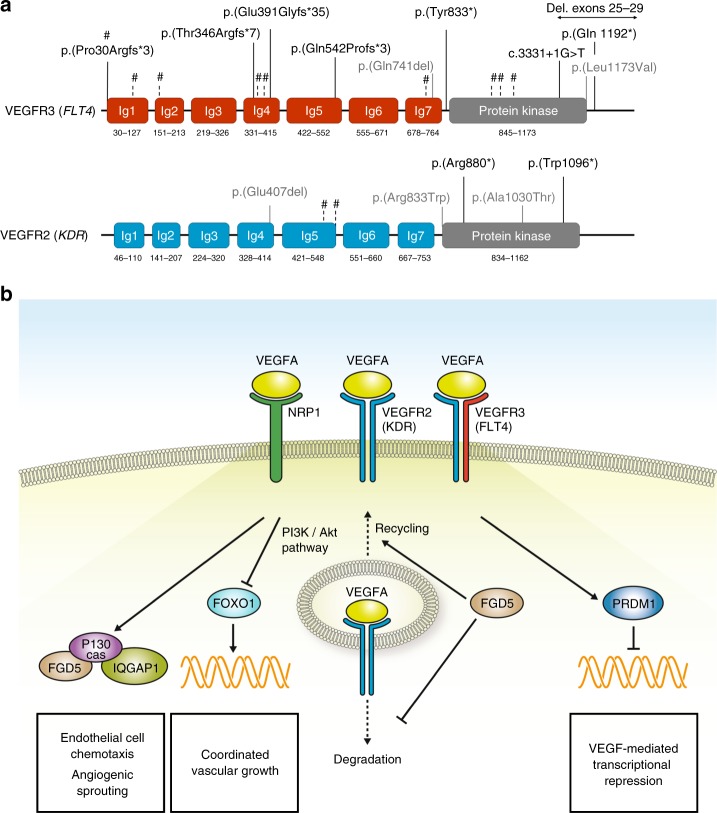
Fig. 1. VEGF pathway and genome sequencing in tetralogy of Fallot.
(a) Variant positions in vascular endothelial growth factor receptors 3 (VEGFR3; FLT4) and 2 (VEGFR2; KDR): loss-of-function variants (black; multiexon 8-kb deletion indicated by horizontal arrow), in-frame deletions or deleterious missense variants (gray). Loss-of-function variants in ref.4 indicated by vertical dashed lines and #; in FLT4 (NM_182925.4), from left to right: p.(Pro30Argfs*3) [1x inherited, 1x de novo], p.(Arg82*), p.(Thr168Serfs*76), p.(Tyr361*), p.(Pro364Alafs*63), p.(Gln736*), p.(Leu935Profs*72), p.(Cys949Argfs*53), p.(Gln999*); and in KDR (NM_002253.2): p.(Lys529*), c.1646-2A>T. Nomenclature as recommended by the Human Genome Variation Society (HGVS; http://varnomen.hgvs.org/). (b) Selected components of vascular endothelial growth factor (VEGF) signaling in endothelial cells, focusing on candidate genes for tetralogy of Fallot and their presumed roles in vascular development. VEGFA induces the formation of VEGFR2 homodimers (blue/blue), VEGFR2/ VEGFR3 heterodimers (blue/red), and binds to the coreceptor NRP1 (ref. 9). VEGFR1 (encoded by FLT1; not shown) may function as a negative regulator for VEGFA signaling, but also forms heterodimers with VEGFR2 (ref. 9). P130cas (encoded by BCAR1) mediates VEGFR2/NRP1 signaling and functions in the assembly of multiprotein complexes, among which are IQGAP1 and FGD5 (ref. 17). FGD5 also inhibits VEGFR2 degradation.18 VEGFR2 suppresses the activity of the transcription factor FOXO1, which is important for the regulation of coordinated vascular sprouting.19 The transcriptional repressor PRDM1 was linked to VEGF signaling in tumor vasculature and in wound healing;20 arterial pole defects in mutant mice indicate PRDM1 also functions in cardiovascular development (Table S5)