Abstract
Background:
Within gastrointestinal malignancies, primary liver (HCC) and pancreatic ductal adenocarcinoma (PDAC), are frequently associated with visceral thromboses (VT). Thrombus formation in the portal (PVT), mesenteric (MVT), or splenic vein (SVT) system leads to portal hypertension and intestinal ischemia. VT in PDAC may convey a risk of increased distal thrombosis and poses therapeutic uncertainty regarding the role of anticoagulation.
Rationale:
An increasing number of reports describe VT associated with PDAC. It is possible that early diagnosis of these events may help reduce morbidity and speculatively improve oncologic outcomes.
Objectives:
Perform a systematic review to study VT (portal, mesenteric and splenic vein thromboses) associated with PDAC and provide a comprehensive review.
Data source:
PubMed, EMBASE, Web of Science, Scopus, and the Cochrane library.
Data extraction and assessment:
Two blinded independent observers extracted and assessed the studies for diagnosis of PVT, MVT, SVT in PDAC. Studies were restricted to English literature published between 2007 and 2016.
Results:
Eleven articles identified. Five case reports and 7 retrospective studies were found with a total of N=127 patients meeting the inclusion criteria. The mean age at diagnosis was 64 years. PVT found in 35% (N= 46), SVT 52% (N= 65), MVT 13% (N= 15). Mean follow up time 26 months. Only 3 of the selected articles studied the impact of anticoagulation in visceral thrombosis. All patients with non-visceral thrombosis (e.g. DVT, PE) were therapeutically treated, in contrast, only rare instances of patients with VT received treatment.
Conclusions:
Visceral thrombosis in PDAC is a frequent finding at diagnosis or during disease progression. Evidence to guide treatment choices is limited and current management is based on inferred experience from non-oncologic settings. Anticoagulation appears to be safe in VT with most of the large studies recommending a careful assessment for patients with high risk of bleeding.
Keywords: Mesenteric vein thrombosis, Pancreas adenocarcinoma, Portal vein Thrombosis, Splenic Vein Thrombosis, Visceral thrombosis, splanchnic vein thrombosis
Graphical Abstract
Background
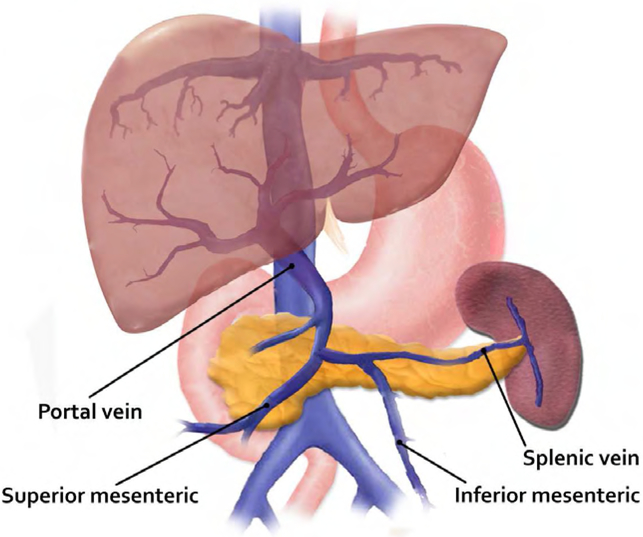
The word splanchnic derives from the Greek “splanchnikos”, from “splanchna”, plural of viscera,(1) thus the term visceral or splanchnic vein thromboses are common adjectives in medical terminology to refer to thromboses in, near or pertaining to the viscera or intestines while visceral relates to the internal organs of the body. In this article we refer to visceral thromboses as the involvement of the abdominal veins, Figure 1 (portal, splenic, mesenteric).
Fig 1.
Anatomic location of the splanchic venous system: Portal Vein, Mesenteric vein(inferior and superior braches) and splenic vein.
Pancreatic ductal adenocarcinoma (PDAC) is currently the fourth leading cause of cancer related death in the USA (2), and one of the most common malignancies associated with thrombotic events (3-6), with an estimated incidence of 36% in a large single-institution cohort of 690 patients with PDAC. (7)Retrospective analyses have found that the most common thrombotic complications include; deep vein thrombosis (dVTE) and pulmonary emboli (PE) both with almost equal incidence (46% vs 39%)(8), catheter-related thrombosis (15%) (9) and visceral thrombosis (VT) predominantly portal vein thrombosis (PVT) (7). With the advancement of diagnostic imaging (10-12), visceral vein thrombosis has become a well-defined entity that is commonly encountered in patients with prothrombotic disorders (28%), cirrhosis (13%) and malignancies such as PDAC and hepatocellular carcinoma. (13). In one study the prevalence of VT in PDAC was approximately 22.9%(14), and almost all were incidentally discovered on routine surveillance and restaging scans(14). With such incidental discoveries and given the lack of therapeutic guidelines for VT management, there are significant uncertainties related to the potential increased risk of complications, reduced survival, and inferior prognosis. (14, 15) In this systematic review we examine the most recent published literature on VT, specifically pertaining to PDAC and assess its clinical implications and prognosis.
Portal Venous System:
Portal Vein Thrombosis (PVT)
PVT is the formation of a thrombus that partially or completely occludes the portal vein, the latter which is formed by the confluence of the splenic vein and the superior mesenteric vein(16). The thrombus can arise in the trunk of the PV and can extend into the intrahepatic branches(17). PVT is the most well studied and described site of visceral thromboses; most notably, due to the increased incidence in patients with liver cirrhosis, inherited thrombophilic disorders and malignancies. The prevalence increases with the severity of cirrhosis, being approximately 1-16% in compensated cirrhosis (18) and 35% in patients with decompensated cirrhosis and HCC. (19,20)
PVT is commonly considered ‘acute’ if presentation is within 60 days or chronic if it has been present for >60 days. However, when chronic it may induce complications due to portal hypertension with worsening ascites, splenomegaly and variceal bleeding. (21)
PVT has been classified into four categories depending on the extent of thrombosis: Type I-limited to PV; Type II-extension into SMV but patent vessels; Type III-diffuse thrombosis of splanchnic venous system with large collaterals; Type IV-extensive splanchnic venous thrombosis but with only fine collaterals.(22). Chronic portal vein obstruction can lead to the development of multiple collateral vessels, known as cavernous transformation.(23)
The long-term sequel of portal vein thrombosis is portal hypertension which in pancreas adenocarcinoma patients has been shown to significantly contribute to ascites formation. A recent study found that 82% of patients with PDAC and ascites had a serum-ascites albumin gradient ≥1.1. In that study the authors concluded that VT and tumor burden were possible causes of increased ascites formation in this population. (24)
Commonly with intra-abdominal diseases, thrombosis will originate in large veins at the site of compression and later progress peripherally to involve smaller vessels, while in contrast, thrombosis due to hematological disorders typically affects the smaller vessels and then progresses to larger trunks.(25)
Mesenteric Vein Thrombosis (MVT)
The mesenteric vein (MV) is anatomically divided into superior and inferior mesenteric veins, in which the former drains into the splenic vein.(16) MVT can be defined as ‘primary’ if not associated with any other disease; and ‘secondary’ if attributed to an acquired etiology (e.g. malignancy or thrombophilia). MVT typically is associated with nonspecific symptoms, may be difficult to diagnosis and has low awareness among clinicians (26). It can also be an uncommon cause of mesenteric ischemia in 5-15% of cases(27). Its prevalence has been reported to be 12%, according to a Swedish autopsy registry study in which 6 of 51 patients had an underlying pancreatic malignancy (28). Common presentations, include presentation with acute abdominal symptoms, particularly in patients with prior thrombotic episodes or documented coagulopathy(29). Currently, MVT is classified as ‘acute’, ‘sub-acute’ and ‘chronic’(30). The former accounts for 6-9% of all cases and typically presents with severe abdominal pain out of proportion to physical exam, whereas chronic MVT, accounts for 20-40% of the cases and is associated with vague and non-specific abdominal discomfort (31).
Splenic Vein Thrombosis (SVT)
Anatomically, the SV arises by the union of smaller vein branches from the stomach, pancreas and large intestine (via the inferior mesenteric vein)(16). It is associated with the posterior surface of the tail and body of the pancreas from the hilum of the spleen to its junction with the superior mesenteric vein, where it forms the portal vein(33). It was originally described as a cause of gastrointestinal bleeding (GIB) (32), but it was later observed to be a frequent thrombosis site owing to: its close vicinity to the pancreas (33), inflammation from pancreatitis (34), direct tumor invasion or compression (35) and as consequence of direct trauma and pseudocyst formation(33).
The foremost hazard of SVT is gastrointestinal bleeding to due to the generation of localized portal hypertension referred as “sinistral”(36) or left-sided” (37). Most of the currently available evidence is connected to pancreatitis-induced SVT(34,38-40). A meta-analysis by Butler et al, found that the overall incidence of SVT in the non-cancer setting was 14.1% and the overall rate of gastrointestinal bleeding was 12.3%. (39) In contrast SVT in PDAC has not been frequently studied and limited data exist. One retrospective study that evaluated SVT in PDAC, by Denadia et al. evaluated N= 70 patients who underwent surgery for pancreatic exocrine cancer. In the cohort, N=27 patients had SVT, and 46% (N=12) had resectable disease (Stage IA/IB, IIA) and 54% had locally advanced disease (IIB/III). PDAC was identified in 19 patients (70%). In this study the authors concluded that SVT was correlated with higher rates of intraoperative blood loss, pancreas-specific complications and reduced long term survival rate (35). This highlights the significance of SVT in the preoperative evaluation of patients with PDAC as well as the potentially increased risk of associated intraoperative complications.
Radiographic Diagnosis
Computed tomography (CT) is the imaging modality of choice for the diagnosis of VT. To make a correct diagnosis, radiologists need to rely on radiographic features to distinguish between bland thrombi (secondary to a hematological disorder) versus tumor thrombus (malignant thrombi) (25), which often can be technically difficult to interpret as no parameter is entirely specific. Several radiographic criteria were proposed by Tublin et al. (41) to differentiate between benign and malignant thrombi (Fig 2-4) including; mural signs (e.g. halo sign, abnormal wall enhancement), vascular signs (e.g. venous filling defect, vein enlargement, venous engorgement) and extramural-nonvascular signs (e.g. mesenteric fat edema, bowel dilation).(25) The precise interpretation of the thrombus etiology has direct therapeutic implications for the oncologic patient.
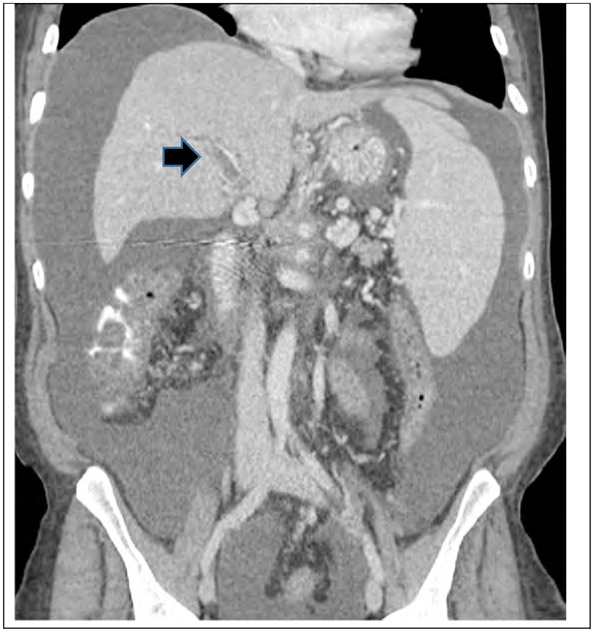
Figure 2.
CT scan with contrast of a 55 year old female patient wtih PDAC who developed portal Vein thrombosis (arrow).
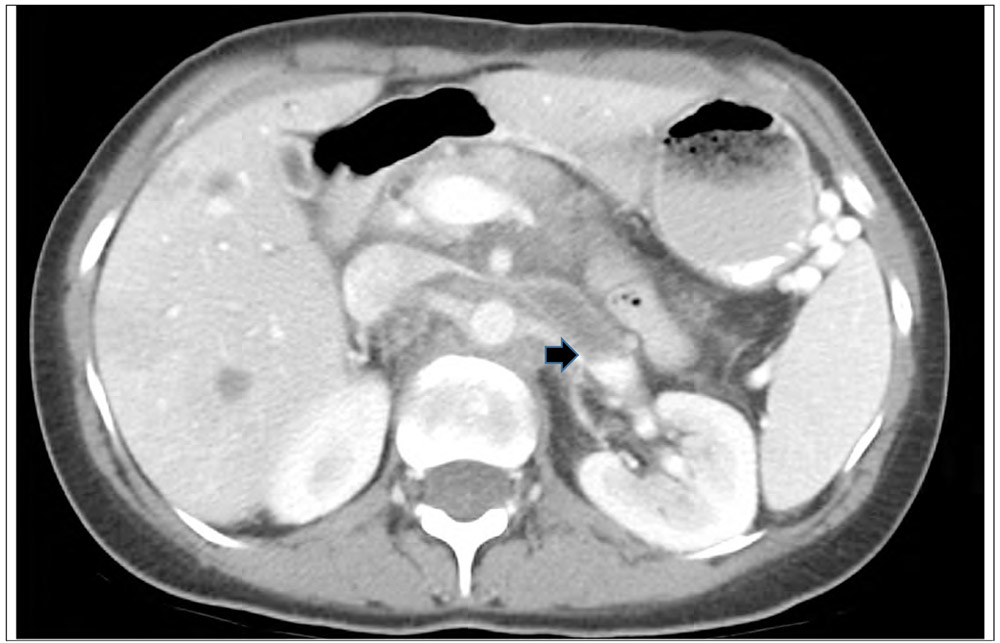
Figure 4.
Ct scan with contrast of a 69 year old male with PDAC, diagnosed with a common iliac vein thrombosis (arrow)
Anticoagulation
The presence of thrombus within the visceral veins may represent a clinical challenge for treatment decisions, particularly in view of the concomitant cancer progression and the prothrombotic state that often accompanies PDAC (42,43). Consequently, this can theoretically lead to intestinal or splenic infarction and thus portend an increased risk of gastrointestinal bleeding with a potential risk of death(44-46). Hence the purpose of anticoagulation is not only thrombus dissolution and recanalization but also to prevent the progression to cavernous transformation of the portal vein and thus a reduced risk of portal hypertension.(23, 47,48).
Current therapeutic strategies follow the recommendations made by the American College of Chest Physicians 9ed (ACCP) (49) for venous thromboembolism, however, evidence from this guideline does not specifically address the use of anticoagulation in circumstances like VT. Use of anticoagulation is not standardized and questions about whether to anticoagulate or not pertain to the type of treatment and duration of therapy and the overall context of prognosis. These uncertainties and the lack of guidelines collectively contribute to challenging decision making in this setting. (50) Currently, most treatment algorithms regarding VT are extrapolated from studies based on patients with liver cirrhosis with/without HCC (13,18,19,51-53), owing to the increased incidence of VT in liver cirrhosis compared to PDAC.
If acute PV, MS or SV thromboses present in patients with malignancy, anticoagulation is generally recommended preferably with either low molecular weight or unfractionated heparin (54,55) given the strong evidence of greater efficacy and safety in contrast to oral vitamin K antagonists (VKA) (56). In the non-cancer setting the duration of treatment should be at least 3 months as per current guidelines by ACCP(49), with goals to halt thrombus extension, achieve vein recanalization, prevent portal hypertension and intestinal ischemia(26), the latter which can be potentially fatal. Currently, expert opinion recommends at least 6 months of LMWH monotherapy.(57). Plessier et al. (46) found that recanalization occurred in one-third of patient receiving early anticoagulation and therefore supported early anticoagulation of patients with acute PVT, nonetheless, if SVT and ascites was detected on imaging, recanalization with anticoagulation was unlikely.
Role of Newer anticoagulants
Following the introduction of the newer oral anticoagulants (NOACs), e.g., dabigatran, apixaban, rivaroxaban, recent data has reported on their application in oncologic patients.(58-61) These novel anticoagulants are attractive to patients and clinicians due to the ease of oral administration and absence of laboratory monitoring(62) making them attractive alternatives to low molecular weight heparin (LMWH). Currently there are limited outcome data on these agents in the cancer setting and there is only one approved reversal agent, idarucizumab (63). Cancer patients pose several unique challenges to AC therapy, primary owed to disease progression that can affect organs (e.g. renal or liver) that can limit therapeutic choices(64) and secondary to anti-cancer therapies that have significant interactions with the CYP3A4 enzyme and/or p-glycoprotein transporter (65,66) which can modify pharmacological clearance resulting in an increased predisposition to bleeding complications.
NOACs have been well studied in acute vein thrombosis. A recent meta-analysis by Sardar et al (59) evaluated the results of six trials with a pooled analysis of 19,832 patients in which 1197 were cancer patients. The authors concluded that NOACs are effective in the prevention and treatment of DVT and did not cause excessive clinically relevant bleeding compared to LMWH for the treatment of acute VTE.
Presently, the use of NOACS in VT is based on expert recommendations. The international society on thrombosis and hemostasis, published in 2013 (65), recommended against the use of NOACs for the initial and long term treatment of cancer associated thrombosis and recommended investigating the efficacy and safety of NOACs in randomized controlled trials. Expert guidance for cancer associated thrombosis published by Khorana et al. (57) and Ageno et al. (67) recommended to consider long term anticoagulation for all patients with visceral thrombi, with treatment decisions to be made on a case-by-case basis by balancing risk factors for recurrence (e.g. underlying prothrombotic conditions) and the risk of bleeding.
Therefore, NOACs appear to be an attractive alternative to provide long-term anticoagulation for VT, however, presently limited evidence cannot recommend their use as standard of care owing to the limited evidence of their safety and efficacy.
METHODS
The primary objective of this systematic literature review was to identify the most recent available evidence pertaining to diagnosis, treatment and management of VT in pancreatic ductal adenocarcinoma. Our hypothesis being that currently there is limited published data pertaining to this topic and clinical management lacks appropriate recommendations. Therefore, we anticipate that this review will increase clinical awareness and encourage further study of visceral thromboses in PDAC. This review followed the elements of the PICOS methodology, shown in table 1.
Table 1 –
Description of the PICO strategy used for study design
| Population | Patients with Pancreas Adenocarcinoma |
| Interest | Portal Vein Thrombosis Splenic Vein Thrombosis Mesenteric Vein Thrombosis |
| Context | Studies published after 2008, that have evaluated patient with visceral thrombosis. |
| Outcome Measure | Appraise the currently literature in this topic. |
Searching Methods
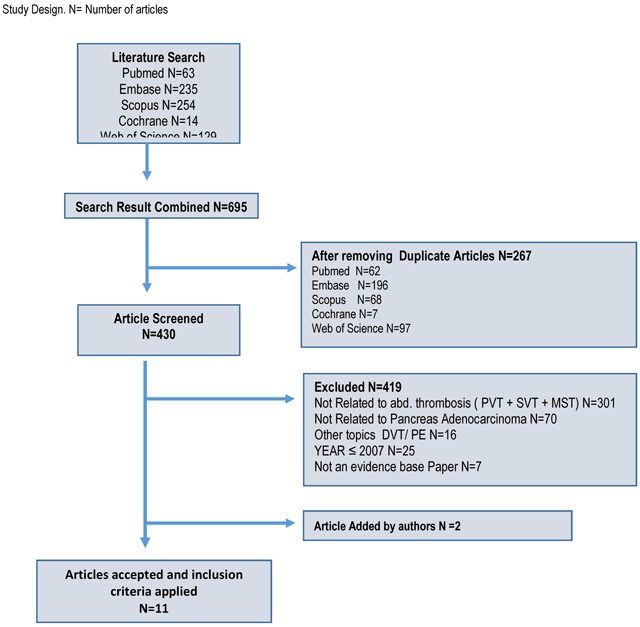
Systematic literature searches were conducted (February 2, 2016) in five databases for references written in English-only with a population age of 19+ years. The searches were filtered to human-only research and include the date range of December 1, 2005 – February 2, 2016. The databases searched were: (1) MEDLINE (via PubMed), (2) Embase, (3) The Cochrane Library (Cochrane), (4) Web of Science (WoS), and (5) Scopus. For the following databases both controlled vocabulary and text words were used in the development of the search strategies: PubMed, Embase, Cochrane. All search results were combined in a bibliographic management tool (EndNote) and duplicates were eliminated both electronically and manually.
The search strategy had two major components that were linked together using the AND operator: (1) pancreatic cancer terms including adenocarcinoma, glandular tumor; (2) intra-abdominal thrombosis terms including portal vein thrombosis, venous thromboembolism, VTE, venous thrombosis, thrombotic events and thromboembolic events. Comprehensive searches were also conducted in two grey literature sources to incorporate this perspective to the final qualitative synthesis of the investigation: (a) Grey Literature Report and (b) Open Grey. For a complete list of MeSH and keyword terms used, please refer to the MEDLINE search strategy accompanying this paper.
Inclusion Criteria and Data extraction
Table 2, depicts the eligibility inclusion criteria. Data extraction was performed by one of the reviewers (AMH) blinded to the names of authors, institutions, journal names of the included studies, and extracted the relevant data. The following information was extracted from each article: authors, year of publication, study type, country of origin, mean age, primary working hypothesis and outcomes. These parameters are presented in the following tables: analysis of included studies and included patients (Table 3) and overall treatment and outcomes from studies (Table 3). Disagreements between the two reviewers (AMH, EOR) were resolved by discussion and analysis of the data. This article is reported in accordance with the guidelines set out by the Preferred Reporting Items for Systematic Reviews and Meta analyses (PRISMA).
Table 2 –
Eligibility Criteria of included studies
| Inclusion Criteria Selected studies were considered eligible if all of the following predefined criteria were met: |
|---|
| a) Studies related to Portal vein, mesenteric vein and/or splenic vein thrombosis |
| b) Pathological confirmation of pancreas Adenocarcinoma |
| c) Year of publication was 2007 or later |
| d) Studies reported in English language |
| e) Full text publications |
Table 3 –
Selected studies evaluating abdominal thromboses in pancreas ductal adenocarcinoma ( N=10 )
| Author/ Year/ Reference |
Study Design |
No of Subjects |
Country | Vein Location |
Age (mean) |
Histology | Primary working hypothesis |
Observations |
|---|---|---|---|---|---|---|---|---|
| Dedania N et al. 2013 | Retrospective study | SVT =19 | USA | Splenic vein | 62.2 | Pancreas adeno carcinoma | SVT is associated with increase intraoperative blood loss, pancreas specific complications and reduced long term survival cross-sectional study to determine the prevalence of asymptomatic (incidental) venous thromboembolism seen on staging CT scans, in a consecutive series of patients. | Retrospective study evaluating surgical complications after distal pancreatectomy in PAC |
| Douma et al. 2015 | Retrospective study | T= 9 PVT=5 SVT=3 RVT=1 |
USA | Portal, Renal and Mesenteric | 57 | Pancreas adeno carcinoma | A second objective was to assess the subsequent therapeutic implications of these thrombi. | |
| Kawaka mi H et al. 2007 | Case report | 1 | Japan | Portal Vein | 68 | Pancreas adeno carcinoma | EUS was helpful to delineate the intraportal growth of the tumor, and ERP to delineate the intraductal growth of the tumor. | tumor with intraductal growth into the main pancreatic duct that presented with tumor thrombus in the portal vein. |
| Menapac e LA et al. 2011 | Retrospective study | T= 45 PVT=18 SVT=14 MVT=13 | USA | Portal, Splenic and Mesenteric | 65.9 | Pancreas adeno carcinoma | Determine the prevalence of both symptomatic and incidental VTE in patients with PAC | Large study that assessed for visceral thrombosis on PAC. Incidentally discovered visceral vein thrombi are associated with worsened mortality and clinical consequences who may benefit from therapeutic anticoagulation. |
| Onesti JK et al. 2013 | Retrospective study | SVT=2 | USA | Splenic Vein | 61 | Pancreas adeno carcinoma | Analysis of the pathologic implication of splenectomy in suspected distal pancreatic malignancy. | Only 2 patients were noted to have splenic vein thrombosis. |
| Roch AM et al. 2015 | Retrospective study | 26 | USA | Splenic Vein | 65.4 | Pancreas adeno carcinoma | Extended distal pancreatectomy for locally advanced adenocarcinoma is associated with a survival benefit. | Single institution retrospective study, long study period from 1996-2011. |
| Roldan-Valadez E et al. 2008 | Case report | 1 | Mexico | Portal Vein | 68 | Pancreas adeno carcinoma | Patient with PAC with clinical findings of portal hypertension who underwent PET/CT with finding of PVT. | PVT did not show enhancement or neovascularity with contrast CT, with the possibility of a bland tumor from altered portal venous hemodynamics. |
| Søgaard KK et all. 2015 | Retrospective study | T= 20 PVT=19 HVT=1 |
Denmark | PVT, HVT | 61 | Pancreas adeno carcinoma | Examined cancer risk after a first time SVT diagnosis, comparing cancer risk with the general Danish population, in addition to comparing survival among patient with and without SVT | Final conclusion: study found evidence that SVT is a strong marker of occult cancer and a predictor of poor prognosis for patients with liver and pancreatic cancer. |
| Venturi A et al. 2007 | Case report | 1 | Italy | Portal vein | 64 | Pancreas adeno carcinoma | CEUS is reliable, non-invasive and useful diagnostic technique in the differential diagnosis between benign and malignant PVT outside the setting of chronic liver disease. | This case report evaluated the role of real-time Contrast-enhance ultrasound(CEUS) in the assessment of PVT |
| Yamato H et al. 2009 | Care Reports | 2 | japan | 1.Portal Vein 2.inferior Mesenteric Vein |
66 | 2: Pancreas tubular Adeno carcinoma | Case 1: underwent surgical resection, 14 months after surgery thrombus extended into SMV, patient was alive 19months after surgery. Case 2: surgical exertion performed, tumor recurrence 4 months after, patient died of liver failure. patient developed acute MVT-PVT on the first postoperative day, was treated with aggressive anticoagulation and early operative thrombectomy with SMV-PV reconstruction. |
Description of three case reports of pancreatic carcinoma, one case was omitted due to tumor histology(endocrine carcinoma) |
| Zyromski NJ et al. 2008 | Case report | 1 | USA | Mesenteric Vein Thrombosis | 69 | Pancreas adeno carcinoma | Case report to express awareness of early postoperative SMV-PV thrombosis after pancreaticoduodenectomy and its catastrophic complications. |
SVT=splenic vein thrombosis(N=62), PVT=portal vein thrombosis(N=41), MVT=mesenteric vein thrombosis(N=15) PAC=pancreas adenocarcinoma, HVT=Hepatic vein thrombosis (N=1) T=total
RESULTS
The flow diagram of the study selection is shown in Fig. 1. A total of 10 articles were identified from the electronic search. After screening the articles, nine articles on VT in PDAC were included and their data was extracted. Two other articles were added after reference search by the authors criteria (68,69). Overall these 11 articles included a total of 127 patients with thromboses (PVT, SVT, MVT). There were no randomized controlled studies. Table 2 shows the characteristics of the included studies; five studies were case reports (70-74), and seven were retrospective studies (14,35,68,69,75-77). All articles, were published after 2007 and were published in the English language. The mean age of patients at diagnosis of all 127 patients was 64.3 years (range: 57-69).
Table 4, denotes some of the studies that assessed treatment and outcomes. Four of the 5 retrospective studies are shown. Median follow up was 26 months. Only two studies evaluated the role of anticoagulation in visceral thromboses. In the study of Menapace et al. (14) 40% of patients were treated with AC, and demonstrated on multivariate analysis that patients with VT had a worsened mortality (HR 2.6, 9% CI 1.6-4.2,p=0.0001), in contrast the use of AC was associated with improved survival (HR 0.30, 95% CI 0.12-74,p=0.009). In another non-randomized study by Søgaard et al.(68) three- month survival after cancer diagnosis was 35% in the group with VT compared to 53% in patients without VT.
Table 4 –
Studies that assessed the treatment modalities and outcomes.
| Author/ Year/ Reference |
Median Follow up |
Anticoagulation | Outcomes | Observations |
|---|---|---|---|---|
| Dedania N et al. 2013 | 28.4 months | Not evaluated | -SVT had a significantly higher rate of pancreas-specific complications, including pancreatic fistula (33 versus 7%, p<0.01) and delayed gastric emptying (15 versus 0%, p<0.02) -Patients without SVT had a trend toward longer median survival(40 versus 20.8 months, p=0.1) |
Group with SVT was younger at 62 years, as compared to 68 years for the non-svt group(p<0.05) |
| Menapace LA et al. 2011 | 36 months | 39.1% of events were treated with AC | -Visceral vein thrombosis on multivariate analysis was associated with worsened mortality (HR2.6, 9% CI 1.6-4.2,p=0.0001) -Use of Anticoagulation was associated with improved survival(HR0.30, 95% CI 0.12-0.74,p=0.009) |
|
| Roch AM et al. 2015 | 24 months follow up |
Not evaluated | -SVT did not significantly affect morbidity, mortality or survival. -Recurrence was not significantly increased in case of splenic vein thrombosis (30.8% vs 38.8%, P = .63), whether it was local recurrence (11.5% P = .63) or distant metastases (19.2% vs 25.4%, P = .6). -Pathologic invasion of the vein did not impact short- and longterm outcomes. |
|
| Søgaard KK et all. 2015 | 18 months | Not evaluated | -3 month survival after cancer diagnosis was 35% for patient with SVT and 53% for patients without SVT. | 2 patients had localized cancer and 11 had advance disease. |
| Zyromski NJ et al. 2008 | 69 yr. (Case report) |
Heparin and thrombectomy | -Resolution of acute MVT-PVT postoperative day after pancreaticoduodenectomy | -Patient became oliguric and tachycardic, and not responding to fluid resuscitation which prompted for abdominal sonogram. |
| Douma et al.2015 | Cross sectional Study | Anticoagulation therapy evaluated | -Authors, observed that patients with DVT and PE were treated with anticoagulants, and none of the patients with VT were treated. | |
| Other pertinent large studies related to visceral thrombosis in patients with or without malignancies | ||||
| Ageno et al. 2015 | 6 years solid Cancer represented 22.7% of cohort |
LMWH=58.6% VKA= 62.4.6% |
- Mean duration of AC was 13. 9 months. - The incidence for major bleeding was 3.9 per 100 patient-years (2.6-6.0) and 5.6 per 100 patient-years (3.9-8.0) for thrombotic events. - Did not find higher rates of hemorrhage between those who received anticoagulants compared with those who did not - most patients (77.0%) received some form of anticoagulant treatment - Supports the safety and efficacy of anticoagulant therapy in most patients with SVT |
Longest multicentric prospective cohort |
| Ageno et al.2014 | 4 years N=12( with only PAC) |
LMWH=58.6% UFH= 4.9% VKA= 31.6% |
- One in four patients with SVT did not receive antithrombotic therapy - Patients with solid cancer and gastrointestinal bleeding are more likely to be left untreated |
This is a large multi centric prospective cohort study with total of 613 patients. |
| Derman et al. 2015 | 2 years | LMWH=20% UFH=12% |
-34% of the PVT group received short-term anticoagulation (< 1 month), specifically LMWH (20%), unfractionated heparin (UFH) (12%), fondaparinux (0.4%), and other therapies (1%). -A total of 64% of the PVT + SPVT and 70% of the PVT + MVT groups received short-term anticoagulation. -6% of the PVT group experienced bleeding complications following diagnosis and intervention. |
Eight patients had pancreas cancer |
SVT=splenic vein thrombosis, PVT=portal vein thrombosis, MVT=mesenteric vein thrombosis PAC=pancreas adenocarcinoma, HVT=Hepatic vein thrombosis
Other pertinent large studies related to VT which involved a diverse cohort are displayed in table 4. The largest studies were by Ageno et al(55,79), who performed a prospective cohort study of 604 patients from 2008-2014 with splanchnic vein thrombosis (22.7% of cohort had solid cancer). This study demonstrated firstly that there were no higher rates of hemorrhage between those who received anticoagulants compared to those who did not (77% of the total cohort was treated with AC) and secondly supported the safety and efficacy of AC in most patients with VT. However, the main endpoint of this study was to assess the incidence of bleeding, mortality and thrombotic events and did not evaluate survival in cancer patients.
DISCUSSION
Visceral thrombosis is an emergent topic in the literature (50,55,67,68,77,79-83). Due to the heterogeneity of presentation, thrombosis can be discovered incidentally through the course of the disease, in particular in patients with advanced metastatic disease, or thrombosed may present with symptoms that lead to further abdominal imaging. With this systematic review and literature analysis we confirmed the significant occurrence of VT in PDAC. However, there are no standard therapeutic guidelines. With recently published studies it has been recognized that VT is a prognostic factor for short-term survival (68) and is a marker of occult cancer (77).
In a retrospective study, Menapace at al.(14) identified from a cohort of 135 patients with PDAC, N=31 (22.9%) had visceral thrombosis. This analysis confirmed that in PDAC, the occurrence of VT was significantly associated with worsened survival (HR 2.6, 1.6-4.2 CI, P <.0001). This finding has clinical importance in the context of disease progression. In the same study patients with advanced disease were strongly correlated with a greater proportion of VT. There were only N= 11 patients with localized disease (IB N= 2, IIB N= 4, III N= 5) and VT, in contrast, N= 32 patients had advanced stage (IV). However, N= 4 of the localized stage underwent pancreatic resection with thrombus involvement.
In PDAC, especially of the head of the pancreas, tumor can frequently invade the superior mesenteric vein and portal vein from direct tumor extension. This vascular invasion is an important parameter for determining surgical resectability. Therefore, it is common, when performing initial staging or subsequent imaging studies to discover thrombi with in the visceral vein. These thrombi can be either bland or tumor-related thrombi, which can sometimes be differentiated by on the basis of CT imaging characteristics.(84) A bland thrombus is defined as a benign filling defect that can be idiopathic or reflect an hypercoagulable state, venous stasis or the presence a foreign body.(85). In contrast, a malignant filling defect or tumor thrombus should be considered when the thrombus itself shows continuity with in the primary mass and enhancement of the filling defect. (85, 86) Additionally, it is important to know that both types of thrombi (bland and tumor) can coexist secondary to the innate hypercoagulable state and from the external compression of a vein (e.g. PV, MV, SV) by the underlying malignancy. (87,88)
Recent research, has explored the significance of incidental visceral thromboses, increasingly discovered during routine CT imaging. (89) Currently, the subcommittee on Hemostasis and Malignancy (90) has recommended use of the term “incidental” and recommended against the use of the term “asymptomatic”. Particularly, because retrospective studies have shown that many of these events are indeed symptomatic, primarily attributed to the malignancy itself.(90)
Evidence supporting therapeutic anticoagulation for incidental VT in cancer patients is not yet clear. In this matter, the ACCP guidelines recommend AC for symptomatic venous thrombosis patients, and no AC for asymptomatic patients with incidentally detected events(49). Conversely, the AASLD, recommends AC in PVT (acute or chronic) and Budd Chiari syndrome. Long-term AC should be reserved for patients with permanent thrombotic risk factors and in the absence of other contraindications(91).
At Memorial Sloan Kettering Cancer Center for patients with an acute visceral thrombosis typically we initiate anticoagulation with a direct oral anticoagulant when the risks are deemed to outweigh the benefits and the patient is otherwise a candidate for active therapy. For patients with chronic thrombosis, the decision to anticoagulate is maded on a case-by-case basis. Of particular significance is the setting of locally advanced/recurrent PDAC where varices may develop related to portal hypertension in the setting of chronic visceral vein thromoboses. These decisions are nuanced and complex and pertain to balancing risks of anticoagulation and bleeding.
Currently, there is no meaningful evidence supporting the use of primary prophylaxis in this setting, however taking into account that most visceral thrombi are diagnosed in advanced stage cancer and there is indirect evidence pointing towards a decrease in survival, anticoagulation should be considered on a case by case basis.
Conclusions
With the aid of increased diagnostic imaging incidental VT is a topical issue in the literature (50,55,67,68,77,79-83). There is ongoing discussion regarding whether AC is indicated or not. Extrapolated evidence from patients with no malignancy has not clearly demonstrated a favorable impact from anticoagulation with low-molecular-weight heparin and improved prognosis. Recommendations are weak as a result of the observational nature of the data, the infrequent finding of visceral thromboses and the limited overall survival in PDAC. Controlled prospective studies are needed to provide the framework of decision making regarding anticoagulation of portal, mesenteric or splenic vein thromboses regarding safety and prevention of longer-term complications. Pending evidence from the use of novel oral anticoagulants will provide an additional choice for management of VT. Moreover, prospective research studies should describe outcomes and management of visceral thrombosis based on vein location, treatment algorithm and cancer stage.
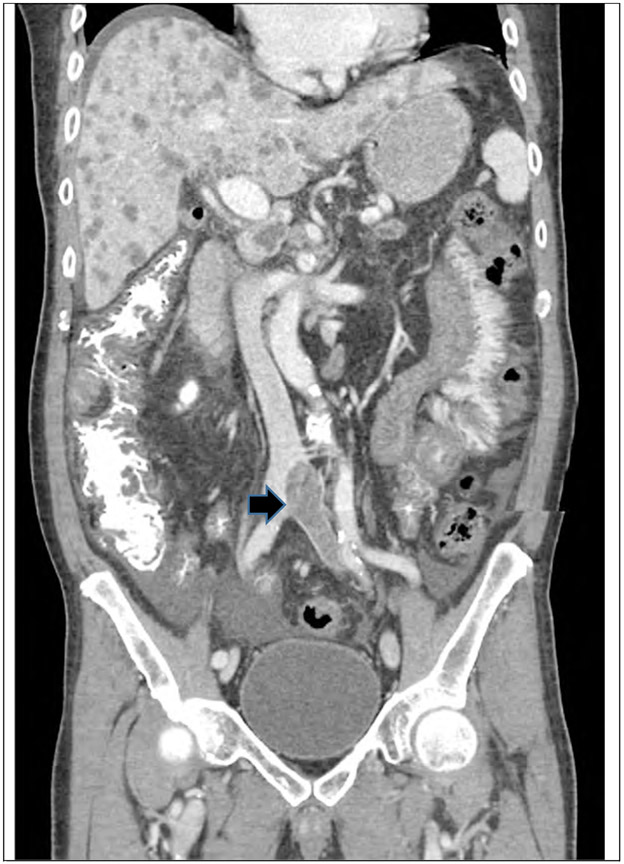
Figure 3.
CT scan with contrast of a 42 year old female with PDAC, diagnosed with renal vein thrombosis (arrow)
Take Home Messages.
Pancreas adenocarcinoma is a well-known risk factor for development of visceral thrombosis.
Visceral thrombosis is a poor prognostic indicator for short term survival (14,83).
In order of incidence, portal vein, splenic and mesenteric vein account for most of the visceral thromboses.
Incidental or non-incidental visceral thromboses should be treated with low-molecular-weight heparin if there are no contraindications (active bleeding, severe thrombocytopenia or end of life care).
The exact role of anticoagulation in the setting of visceral thromboses with regard to oncologic outcome and prevention of complications remains to be fully defined.
Acknowledgments
Funding acknowledgements: P30 CA008748 - Cancer Center Support Grant
Footnotes
Conflict(s) of Interest/Disclosures (s): None of the authors have any financial or other relations that could lead to a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lisowski FP, Oxnard CE. Anatomical Terms And Their Derivation. River Edge, SG: World Scientific; 2007. [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015. January 5;65(1):5–29. [DOI] [PubMed] [Google Scholar]
- 3.Dumitrascu DL, Suciu O, Grad C, Gheban D. Thrombotic Complications of Pancreatic Cancer: Classical Knowledge Revisited. Dig Dis. 2010;28(2):350–4. [DOI] [PubMed] [Google Scholar]
- 4.Shah MM, Saif MW. Pancreatic cancer and thrombosis. Highlights from the “2010 ASCO Annual Meeting.” Chicago, IL, USA June 4-8, 2010 JOP; 2010;11(4):331–3. [PubMed] [Google Scholar]
- 5.Epstein AS, O'Reilly EM. Exocrine pancreas cancer and thromboembolic events: a systematic literature review. J Natl Compr Canc Netw. 2012. July 1;10(7):835–46. [DOI] [PubMed] [Google Scholar]
- 6.Levine MN, Lee AY, Kakkar AK. From Trousseau to targeted therapy: new insights and innovations in thrombosis and cancer J Thromb Haemost. Blackwell Science Inc; 2003. July 1;1(7):1456–63. [DOI] [PubMed] [Google Scholar]
- 7.Epstein AS, Soff GA, Capanu M, Crosbie C, Shah MA, Kelsen DP, et al. Analysis of incidence and clinical outcomes in patients with thromboembolic events and invasive exocrine pancreatic cancer. Cancer. 2011. October 11;118(12):3053–61. [DOI] [PubMed] [Google Scholar]
- 8.Freni SC, Wiepjes GJ. Preparing cell suspensions from cervical smears with pepsine and ultrasonic treatment. Acta Cytol. 1975. May;19(3):306–12. [PubMed] [Google Scholar]
- 9.Ouaissi M, Frasconi C, Mege D, Panicot-Dubois L, Boiron L, Dahan L, et al. Impact of venous thromboembolism on the natural history of pancreatic adenocarcinoma. HBPD INT. 2015. August;14(4):436–42. [DOI] [PubMed] [Google Scholar]
- 10.Surasi DS, O'Malley JP, Bhambhvani P. 18F-FDG PET/CT Findings in Portal Vein Thrombosis and Liver Metastases. J Nucl Med Technol. 2015. September 3;43(3):229–30. [DOI] [PubMed] [Google Scholar]
- 11.Alexandrescu L, Dumitru E, Tofolean IT, Suceveanu A, Moise M. Characterization of Portal Vein Thrombus With the Use of Contrast-Enhanced Sonography. J Hepatol. 2006. August 16;52:1–6. [Google Scholar]
- 12.Lee H-K, Park SJ, Yi B-H, Yeon E-K, Kim JH, Hong H-S. Portal vein thrombosis: CT features. Abdom Imaging. 2007. August 12;33(1):72–9. [DOI] [PubMed] [Google Scholar]
- 13.Søgaard KK, Astrup LB, Vilstrup H, Gronbaek H. Portal vein thrombosis; risk factors, clinical presentation and treatment BMC Gastroenterology. BioMed Central Ltd; 2007. August 15;7(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menapace LA, Peterson DR, Berry A, Sousou T, Khorana AA. Symptomatic and incidental thromboembolism are both associated with mortality in pancreatic cancer. Thromb Haemost. 2011 ed. Schattauer Publishers; 2011. August;106(2):371–8. [DOI] [PubMed] [Google Scholar]
- 15.Shaib W, Deng Y, Zilterman D, Lundberg B, Saif MW. Assessing risk and mortality of venous thromboembolism in pancreatic cancer patients. Anticancer Res. 2010. October;30(10):4261–4. [PubMed] [Google Scholar]
- 16.Cheng EYEA. “Liver” Schwartz's Principles of Surgery, 10e. Eds. Brunicardi F. Charles, et al. New York, NY: McGraw-Hill, 2014. [Internet]. accessmedicine.mhmedical.com.libproxy1.usc.edu. [cited 2016 Mar 31]. Available from: http://accessmedicine.mhmedical.com.libproxy1.usc.edu/content.aspx?sectionid=59610873&bookid=980&jumpsectionID=100401051&Resultclick=2 [Google Scholar]
- 17.Chawla YK, Bodh V. Portal Vein Thrombosis Journal of Clinical and Experimental Hepatology. Elsevier Ltd; 9999 January 1;5(1):22–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okuda K, Ohnishi K, Kimura K, Matsutani S, Sumida M, Goto N, et al. Incidence of portal vein thrombosis in liver cirrhosis. An angiographic study in 708 patients. Gastroenterology. 1985. August;89(2):279–86. [DOI] [PubMed] [Google Scholar]
- 19.Amitrano L, Anna Guardascione M, Brancaccio V, Margaglione M, Manguso F, Iannaccone L, et al. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J Hepatol. 2004. May;40(5):736–41. [DOI] [PubMed] [Google Scholar]
- 20.Belli L, Romani F, Sansalone CV, Aseni P, Rondinara G. Portal thrombosis in cirrhotics. A retrospective analysis Ann Surg. Lippincott, Williams, and Wilkins; 1986. March;203(3):286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chawla Y, Duseja A, Dhiman RK. Review article: the modern management of portal vein thrombosis Alimentary pharmacology & therapeutics. Blackwell Publishing Ltd; 2009. November 1;30(9):881–94. [DOI] [PubMed] [Google Scholar]
- 22.Jamieson NV. CHANGING PERSPECTIVES IN PORTAL VEIN THROMBOSIS AND LIVER TRANSPLANTATION. Transplantation. 2000. May 15;69(9):1772. [DOI] [PubMed] [Google Scholar]
- 23.Vilgrain V, Condat B, O'Toole D, Plessier A, Ruszniewski P, Valla DC. Pancreatic portal cavernoma in patients with cavernous transformation of the portal vein: MR findings. Eur Radiol. 2009. November;19(11):2608–13. [DOI] [PubMed] [Google Scholar]
- 24.Mier Hicks, Chou J, Capanu M, Lowery MA, Yu KH, O'Reilly EM. Pancreas Adenocarcinoma: Ascites, Clinical Manifestations, and Management Implications. Clin Colorectal Cancer. 2016. May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duran R, Denys AL, Letovanec I, Meuli RA, Schmidt S. Multidetector CT features of mesenteric vein thrombosis. RadioGraphics. 2012. September;32(5):1503–22. [DOI] [PubMed] [Google Scholar]
- 26.Acosta S, Ogren M, Sternby NH, Bergqvist D. Mesenteric venous thrombosis with transmural intestinal infarction: a population-based study. Journal of vascular ... 2005. [DOI] [PubMed] [Google Scholar]
- 27.Grendell JH, Ockner RK. Mesenteric venous thrombosis. Gastroenterology. 1982. February;82(2):358–72. [PubMed] [Google Scholar]
- 28.Acosta S, Alhadad A, Svensson P, Ekberg O. Epidemiology, risk and prognostic factors in mesenteric venous thrombosis Br J Surg. John Wiley & Sons, Ltd; 2008. October 1;95(10):1245–51. [DOI] [PubMed] [Google Scholar]
- 29.Morasch MD, Ebaugh JL, Chiou AC, Matsumura JS, Pearce WH, Yao JST. Mesenteric venous thrombosis: A changing clinical entity. J Vasc Surg. 2001. October;34(4):680–4. [DOI] [PubMed] [Google Scholar]
- 30.Bayraktar Y, Harmanci O. Etiology and consequences of thrombosis in abdominal vessels. World J Gastroenterol. 2006. February 28;12(8):1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hmoud B, Singal AK, Kamath PS. Mesenteric Venous Thrombosis. Journal of Clinical and Experimental Hepatology. Elsevier Ltd; 2014. September 1 ;4(3):257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirschfeld DMH. Die Erkrankungen der Milz. In: Die Erkrankungen der Milz. Berlin, Heidelberg: Springer Berlin Heidelberg; 1920. pp. 1–77. [Google Scholar]
- 33.Mulholland MW, Lillemoe KD, Doherty GM, Maier RV. Greenfield's Surgery: Scientific Principles & Practice. 2012. [Google Scholar]
- 34.Heider TR, Azeem S, Galanko JA, Behrns KE. The natural history of pancreatitis-induced splenic vein thrombosis. Ann Surg. 2004. June;239(6):876–80-discussion880–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dedania N, Agrawal N, Winter JM, Koniaris LG, Rosato EL, Sauter PK, et al. Splenic Vein Thrombosis Is Associated with an Increase in Pancreas-Specific Complications and Reduced Survival in Patients Undergoing Distal Pancreatectomy for Pancreatic Exocrine Cancer. J Gastrointest Surg. 2013. June 25;17(8):1392–8. [DOI] [PubMed] [Google Scholar]
- 36.Turrill FL, Mikkelsen WP. Sinistral (Left-Sided) Extrahepatic Portal Hypertension Arch Surg. American Medical Association; 1969. September 1;99(3):365–8. [DOI] [PubMed] [Google Scholar]
- 37.Little AG, Moossa AR. Gastrointestinal hemorrhage from left-sided portal hypertension: An unappreciated complication of pancreatitis The American Journal of Surgery. Elsevier; 1981. January 1;141(1):153–8. [DOI] [PubMed] [Google Scholar]
- 38.Belli AM, Jennings CM, Nakielny RA. Splenic and portal venous thrombosis: a vascular complication of pancreatic disease demonstrated on computed tomography. Clinical radiology. 1990. [DOI] [PubMed] [Google Scholar]
- 39.Butler JR, Eckert GJ, Zyromski NJ, Leonardi MJ, Lillemoe KD, Howard TJ. Natural history of pancreatitis-induced splenic vein thrombosis: a systematic review and meta-analysis of its incidence and rate of gastrointestinal bleeding MHPB. Elsevier Masson SAS; 2011. January 1;13(12):839–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parikh S, Shah R, Kapoor P. Portal Vein Thrombosis. The American Journal of Medicine. 2010. February;123(2):111–9. [DOI] [PubMed] [Google Scholar]
- 41.Tublin ME, Dodd GD, Baron RL. Benign and malignant portal vein thrombosis: differentiation by CT characteristics. AJR Am J Roentgenol. 1997. March;168(3):719–23. [DOI] [PubMed] [Google Scholar]
- 42.Afsar CU, Gunaldi M, Kum P, Sahin B, Erkisi M, Kara IO, et al. Pancreatic carcinoma, thrombosis and mean platelet volume: single center experience from the southeast region of Turkey. Asian Pac J Cancer Prev. 2014;15(21):9143–6. [DOI] [PubMed] [Google Scholar]
- 43.Khorana AA, Fine RL. Pancreatic cancer and thromboembolic disease. The Lancet Oncology. 2004. November;5(11):655–63. [DOI] [PubMed] [Google Scholar]
- 44.Condat B, Pessione F, Hillaire S, Denninger M-H, Guillin M-C, Poliquin M, et al. Current outcome of portal vein thrombosis in adults: Risk and benefit of anticoagulant therapy. Gastroenterology. 2001. February;120(2):490–7. [DOI] [PubMed] [Google Scholar]
- 45.Connolly GC, Chen R, Hyrien O, Mantry P, Bozorgzadeh A, Abt P, et al. Incidence, risk factors and consequences of portal vein and systemic thromboses in hepatocellular carcinoma. Thromb Res [Internet]. 2008;122(3):299–306. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18045666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plessier A, Darwish-Murad S, Hernandez-Guerra M, Consigny Y, Fabris F, Trebicka J, et al. Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology. 2010. January;51(1):210–8. [DOI] [PubMed] [Google Scholar]
- 47.Condat B, Pessione F, Helene Denninger M, Hillaire S, Valla D. Recent portal or mesenteric venous thrombosis: increased recognition and frequent recanalization on anticoagulant therapy. Hepatology. 2000. September;32(3):466–70. [DOI] [PubMed] [Google Scholar]
- 48.Turnes J, Pagan JCG, Gonzalez M, Aracil C, Calleja JL, Ripoll C, et al. Portal Hypertension–Related Complications After Acute Portal Vein Thrombosis: Impact of Early Anticoagulation Clinical Gastroenterology and Hepatology. Elsevier; 2008. December 1;6(12):1412–7. [DOI] [PubMed] [Google Scholar]
- 49.Kearon C, Akl Elie A, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic Therapy for VTE Disease. CHEST. 2012. February;141(2):e419S–e496S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kreuziger LB, Ageno W, Lee A. Management of incidental splanchnic vein thrombosis in cancer patients. Hematology. 2014. December 5;2014(1):318–20. [DOI] [PubMed] [Google Scholar]
- 51.Rössle M, Bausch B, Klinger C. Therapy Algorithm for Portal Vein Thrombosis in Liver Cirrhosis: The Internist's Point of View. Viszeralmedizin. 2014. December;30(6):401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nery F, Chevret S, Condat B, de Raucourt E, Boudaoud L, Rautou P-E, et al. Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: results of a longitudinal study. Hepatology. 2015. February;61 (2):660–7. [DOI] [PubMed] [Google Scholar]
- 53.Janssen HL, Wijnhoud A, Haagsma EB, van Uum SH, van Nieuwkerk CM, Adang RP, et al. Extrahepatic portal vein thrombosis: aetiology and determinants of survival Gut. BMJ Publishing Group Ltd and British Society of Gastroenterology; 2001. November;49(5):720–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ageno W, Dentali F, Squizzato A. How I treat splanchnic vein thrombosis. Blood. 2014. [DOI] [PubMed] [Google Scholar]
- 55.Ageno W, Riva N, Schulman S, Bang SM, Sartori MT, Grandone E, et al. Antithrombotic treatment of splanchnic vein thrombosis: results of an international registry. Semin Thromb Hemost. 2014. February;40(1):99–105. [DOI] [PubMed] [Google Scholar]
- 56.Lee AYY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003. July 10;349(2):146–53. [DOI] [PubMed] [Google Scholar]
- 57.Khorana AA, Carrier M, Garcia DA, Lee AYY. Guidance for the prevention and treatment of cancer-associated venous thromboembolism J Thromb Thrombolysis. Springer US; 2016. January 5;41(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Franchini M, Bonfanti C, Lippi G. Cancer-associated thrombosis: investigating the role of new oral anticoagulants. Thromb Res. 2015. [DOI] [PubMed] [Google Scholar]
- 59.Sardar P, Chatterjee S, Herzog E, Pekler G, Mushiyev S, Pastori LJ, et al. New oral anticoagulants in patients with cancer: current state of evidence. Am J Ther. 2015. November;22(6):460–8. [DOI] [PubMed] [Google Scholar]
- 60.Hulle T, Exter PL, Kooiman J. Meta-analysis of the efficacy and safety of new oral anticoagulants in patients with cancer-associated acute venous thromboembolism. … of Thrombosis and ... 2014. [DOI] [PubMed] [Google Scholar]
- 61.Wharin C, Tagalakis V. Management of venous thromboembolism in cancer patients and the role of the new oral anticoagulants. Blood Reviews. 2014. [DOI] [PubMed] [Google Scholar]
- 62.Short NJ, Connors JM. New oral anticoagulants and the cancer patient. The Oncologist. 2014. January;19(1):82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Glund S, Moschetti V, Norris S, Stangier J. A randomised study in healthy volunteers to investigate the safety, tolerability and pharmacokinetics of idarucizumab, a specific antidote to dabigatran. Thrombosis & ... 2015. [DOI] [PubMed] [Google Scholar]
- 64.Franchini M, Bonfanti C, Lippi G. Cancer-associated thrombosis: investigating the role of new oral anticoagulants Thromb Res. Elsevier Ltd; 2015. May 1;135(5):777–81. [DOI] [PubMed] [Google Scholar]
- 65.Carrier M, Khorana AA, Zwicker JI, Noble S, Lee AYY, on behalf of the subcommittee on Haemostasis and Malignancy for the SSC of the ISTH. Management of challenging cases of patients with cancer-associated thrombosis including recurrent thrombosis and bleeding: guidance from the SSC of the ISTH. J Thromb Haemost. 2013. September 12;11(9):1760–5. [DOI] [PubMed] [Google Scholar]
- 66.Lee A, Carrier M. Treatment of cancer-associated thrombosis: perspectives on the use of novel oral anticoagulants. Thromb Res. 2014. [DOI] [PubMed] [Google Scholar]
- 67.Ageno W, Beyer-Westendorf J, Garcia DA, Lazo-Langner A, McBane RD, Paciaroni M. Guidance for the management of venous thrombosis in unusual sites J Thromb Thrombolysis. Springer US; 2016. September 5;41(1):129–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sogaard KK, Farkas DK, Pedersen L, Sørensen HT. Splanchnic venous thrombosis is a marker of cancer and a prognostic factor for cancer survival. Blood. 2015. [DOI] [PubMed] [Google Scholar]
- 69.Douma RA, Kok MGM, Verberne LM, Kamphuisen PW, Büller HR. Incidental venous thromboembolism in cancer patients: prevalence and consequence Thromb Res. Elsevier Ltd; 2010. June 1;125(6):e306–9. [DOI] [PubMed] [Google Scholar]
- 70.Kawakami H, Kuwatani M, Hirano S, Kondo S. Pancreatic endocrine tumors with intraductal growth into the main pancreatic duct and tumor thrombus within the portal vein: a case report and review of the literature. Intern Med. 2007. [DOI] [PubMed] [Google Scholar]
- 71.Roldan-Valadez E, Ortega-Lopez N, Valdivieso-Cardenas G, Vega-Gonzalez I, Herrera-Gomez A. (18)F-FDG PET/CT for discrimination between tumor extension and blood thrombus in pancreatic adenocarcinoma associated with portal vein thrombosis. Rev Esp Med Nucl. 2008. January;27(1):40–4. [DOI] [PubMed] [Google Scholar]
- 72.Venturi A, Piscaglia F, Silvagni E, Righini R, Fabbrizio B, Cescon M, et al. Role of real-time contrast-enhanced ultrasound in the assessment of metastatic portal vein thrombosis. Ultraschall in der Medizin (Stuttgart, Germany : 1980). 2007 ed. 2007. February;28(1):75–8. [DOI] [PubMed] [Google Scholar]
- 73.Yamato H, Kawakami H, Kuwatani M, Shinada K. Pancreatic carcinoma associated with portal vein tumor thrombus: three case reports. Intern Med. 2009. [DOI] [PubMed] [Google Scholar]
- 74.Zyromski NJ, Howard TJ. Acute superior mesenteric—portal vein thrombosis after pancreaticoduodenectomy: treatment by operative thrombectomy. Surgery. 2008. April;143(4):566–7. [DOI] [PubMed] [Google Scholar]
- 75.Roch AM, Singh H, Turner AP, Ceppa EP, House MG, Zyromski NJ, et al. Extended distal pancreatectomy for pancreatic adenocarcinoma with splenic vein thrombosis and/or adjacent organ invasion. Am J Surg. 2015. March;209(3):564–9. [DOI] [PubMed] [Google Scholar]
- 76.Onesti JK, Chung MH, Jain DH, Stafford MM, Attawala PP. A review of splenic pathology in distal pancreatectomies Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al. ]. Elsevier India, a division of Reed Elsevier India Pvt. Ltd; 2013. November 12;13(6):625–8. [DOI] [PubMed] [Google Scholar]
- 77.Derman BA, Kwaan HC. Risk Factors, Diagnosis, Management, and Outcome of Splanchnic Vein Thrombosis: A Retrospective Analysis. Semin Thromb Hemost. 2015. July;41(5):503–13. [DOI] [PubMed] [Google Scholar]
- 78.Yamamoto Y, Ikoma H, Morimura R, Konishi H, Murayama Y, Komatsu S, et al. Optimal duration of the early and late recurrence of pancreatic cancer after pancreatectomy based on the difference in the prognosis. Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al. ]. 2014. Nov-Dec;14(6):524–9. [DOI] [PubMed] [Google Scholar]
- 79.Ageno W, Riva N, Schulman S, Beyer-Westendorf J, Bang SM, Senzolo M, et al. Long-term Clinical Outcomes of Splanchnic Vein Thrombosis: Results of an International Registry. JAMA Intern Med. 2015. September;175(9):1474–80. [DOI] [PubMed] [Google Scholar]
- 80.Riva N, Donadini MP, Dentali F, Squizzato A, Ageno W. Clinical approach to splanchnic vein thrombosis: Risk factors and treatment Thromb Res. Elsevier Ltd; 2012. October 1;130(S1):S1–S3. [DOI] [PubMed] [Google Scholar]
- 81.Donadini MP, Dentali F, Ageno W. Splanchnic vein thrombosis: new risk factors and management Thromb Res. Elsevier Ltd; 2012. May 7;129(S1):S93–6. [DOI] [PubMed] [Google Scholar]
- 82.Valla D Splanchnic Vein Thrombosis. Semin Thromb Hemost. 2015. July;41(5):494–502. [DOI] [PubMed] [Google Scholar]
- 83.Thatipelli MR, McBane RD, Hodge DO, Wysokinski WE. Survival and Recurrence in Patients With Splanchnic Vein Thromboses. Clinical Gastroenterology and Hepatology. 2010. February;8(2):200–5. [DOI] [PubMed] [Google Scholar]
- 84.Tublin ME, Dodd GD 3, Baron RL. Benign and malignant portal vein thrombosis: differentiation by CT characteristics. AJR Am J Roentgenol. American Public Health Association; 2013. January 19. [DOI] [PubMed] [Google Scholar]
- 85.Kaufman LB, Yeh BM, Breiman RS, Joe BN, Qayyum A, Coakley FV. Inferior Vena Cava Filling Defects on CT and MRI. American Journal of Roentgenology. American Roentgen Ray Society; 2012. November 23. [DOI] [PubMed] [Google Scholar]
- 86.Smillie RP, Shetty M, Boyer AC, Madrazo B, Jafri SZ. Imaging Evaluation of the Inferior Vena Cava. RadioGraphics. Radiological Society of North America; 2015. March 12. [DOI] [PubMed] [Google Scholar]
- 87.Shah ZK, McKernan MG, Hahn PF. Enhancing and expansile portal vein thrombosis: value in the diagnosis of hepatocellular carcinoma in patients with multiple hepatic lesions. American Journal of ... 2007. [DOI] [PubMed] [Google Scholar]
- 88.Shah MM, Saif MW. Pancreatic cancer and thrombosis. Journal of the Pancreas. 2010;11(4):331–3. [PubMed] [Google Scholar]
- 89.Singh R, Sousou T, Mohile S. High rates of symptomatic and incidental thromboembolic events in gastrointestinal cancer patients. Journal of Thrombosis ... 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khorana AA, O’CONNELL C, Agnelli G, Liebman HA, Lee AYY. Incidental venous thromboembolism in oncology patients. Journal of Thrombosis and Haemostasis. Blackwell Publishing Ltd; 2012. December 1;10(12):2602–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.DeLeve LD, VALLA DC, Garcia Tsao G Vascular disorders of the liver Hepatology. Wiley Subscription Services, Inc., A Wiley Company; 2009. May 1;49(5):1729–64. [DOI] [PMC free article] [PubMed] [Google Scholar]