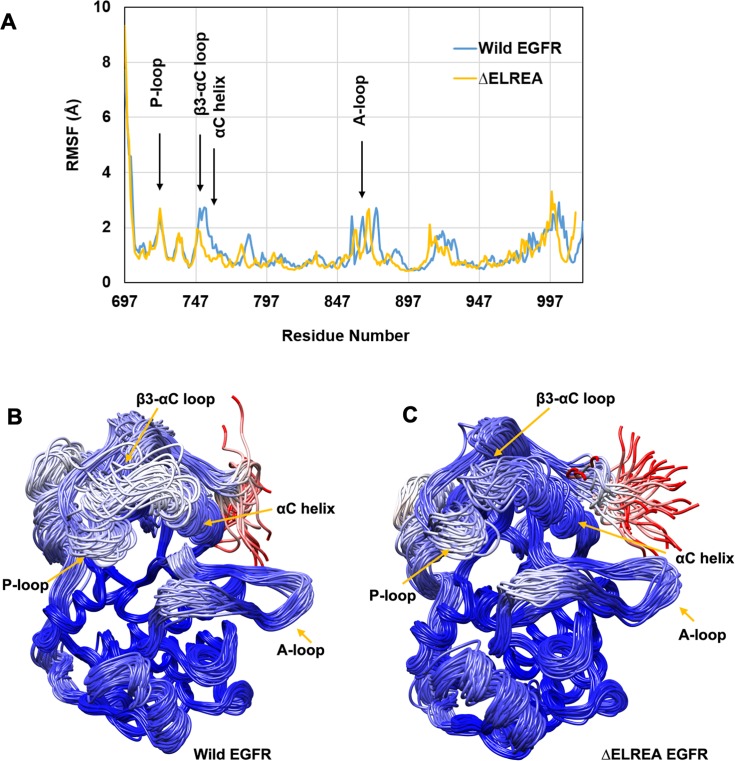
Fig 4. Cα-atom fluctuations and conformational ensemble of active wild-type and ΔELREA EGFRs.
(A) RMSF calculated over Cα atoms for conformations sampled from MDS, showing large fluctuations for unstructured regions in both EGFRs; wild-type (Wild EGFR) and mutant (ΔELREA) EGFR are offset by 5 residues starting from residue 746. Superimposed conformations of wild-type (B) and ΔELREA (C). Chain traces are colored based on RMSDs for individual Cα atoms within the ensemble according to the program Chimera: The gradient of coloring varies from blue with RMSD = 0.45 Å (minimum observed value), to white at 5.6 Å and to red = 11 Å (maximum observed value).